Radiological Staging of Thyroid-Associated Ophthalmopathy: Comparison of T1 Mapping with Conventional MRI
- PMID: 33144856
- PMCID: PMC7599391
- DOI: 10.1155/2020/2575710
Radiological Staging of Thyroid-Associated Ophthalmopathy: Comparison of T1 Mapping with Conventional MRI
Abstract
Background: Accurate staging of patients with thyroid-associated ophthalmopathy (TAO) is crucial for clinical decision. Full cognition of pathologic changes and staging TAO using conventional T2-weighted imaging is still limited.
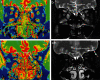
Purpose: To investigate the feasibility of using T1 mapping to evaluate changes of extraocular muscles (EOMs) in TAO patients, as well as to compare T1 mapping and conventional T2-weighted imaging in staging TAO.
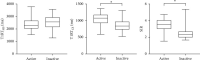
Materials and methods: Forty TAO patients were retrospectively enrolled. "Hot spot" and "cold spot" T1 relaxation times (T1RTHS and T1RTCS) of EOMs, as well as conventionally applied highest signal intensity ratio (SIR) of EOMs, were measured and compared between active and inactive groups.
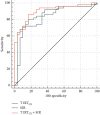
Results: T1RTCS and SIR were significantly higher in active TAOs than in the inactive ones (P < 0.001), while T1RTHS was not (P=0.093). Meanwhile, T1RTCS and SIR were positively correlated with clinical activity score (r = 0.489, 0.540; P < 0.001). TIRTCS and SIR showed no significant area under curve for staging TAO (0.830 vs. 0.852; P=0.748). T1RTCS ≥ 1000 alone showed optimal staging specificity (90.0%), while integration of T1RTCS ≥ 1000 and SIR ≥ 2.9 demonstrated optimal staging efficiency and sensitivity (area under curve, 0.900; sensitivity, 86.0%).
Conclusions: Our findings suggest that the T1-mapping technique holds the potency to be utilized in TAO. The derived T1RTCS of EOMs, which may be associated with fat infiltration, could be a useful biomarker to stage the disease, serving added efficiency, sensitivity, and specificity to single usage of conventional SIR.
Copyright © 2020 Lu Chen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Usefulness of two-point Dixon T2-weighted imaging in thyroid-associated ophthalmopathy: comparison with conventional fat saturation imaging in fat suppression quality and staging performance.Br J Radiol. 2021 Feb 1;94(1118):20200884. doi: 10.1259/bjr.20200884. Epub 2020 Dec 22. Br J Radiol. 2021. PMID: 33353397 Free PMC article.
-
Dixon MRI-based quantitative parameters of extraocular muscles, intraorbital fat, and lacrimal glands for staging thyroid-associated ophthalmopathy.Insights Imaging. 2024 Jun 9;15(1):136. doi: 10.1186/s13244-024-01693-w. Insights Imaging. 2024. PMID: 38853188 Free PMC article.
-
Quantitative T1 mapping MRI for the assessment of extraocular muscle fibrosis in thyroid-associated ophthalmopathy.Endocrine. 2022 Feb;75(2):456-464. doi: 10.1007/s12020-021-02873-0. Epub 2021 Sep 21. Endocrine. 2022. PMID: 34549377
-
The Value of MRI and Radiomics for the Diagnostic Evaluation of Thyroid-Associated Ophthalmopathy.Diagnostics (Basel). 2025 Feb 6;15(3):388. doi: 10.3390/diagnostics15030388. Diagnostics (Basel). 2025. PMID: 39941318 Free PMC article. Review.
-
Clinical Management and Therapeutic Strategies for the Thyroid-Associated Ophthalmopathy: Current and Future Perspectives.Curr Eye Res. 2020 Nov;45(11):1325-1341. doi: 10.1080/02713683.2020.1776331. Epub 2020 Jun 21. Curr Eye Res. 2020. PMID: 32567373 Review.
Cited by
-
Histogram of Apparent Diffusion Coefficient to Evaluate the Activity of Thyroid-Associated Ophthalmopathy.Immun Inflamm Dis. 2025 Jan;13(1):e70131. doi: 10.1002/iid3.70131. Immun Inflamm Dis. 2025. PMID: 39835877 Free PMC article.
-
Diffusion Tensor Imaging Technology to Quantitatively Assess Abnormal Changes in Patients With Thyroid-Associated Ophthalmopathy.Front Hum Neurosci. 2022 Feb 4;15:805945. doi: 10.3389/fnhum.2021.805945. eCollection 2021. Front Hum Neurosci. 2022. PMID: 35185495 Free PMC article.
-
Quantitative assessment of extraocular muscles in Graves' ophthalmopathy using T1 mapping.Eur Radiol. 2023 Dec;33(12):9074-9083. doi: 10.1007/s00330-023-09931-3. Epub 2023 Jul 19. Eur Radiol. 2023. PMID: 37466707
-
Computed tomography and magnetic resonance imaging approaches to Graves' ophthalmopathy: a narrative review.Front Endocrinol (Lausanne). 2024 Jan 8;14:1277961. doi: 10.3389/fendo.2023.1277961. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38260158 Free PMC article. Review.
-
A systematic review of multimodal clinical biomarkers in the management of thyroid eye disease.Rev Endocr Metab Disord. 2022 Jun;23(3):541-567. doi: 10.1007/s11154-021-09702-9. Epub 2022 Jan 23. Rev Endocr Metab Disord. 2022. PMID: 35066781
References
LinkOut - more resources
Full Text Sources

