Triple knockdown of CDC37, HSP90-alpha and HSP90-beta diminishes extracellular vesicles-driven malignancy events and macrophage M2 polarization in oral cancer
- PMID: 33144925
- PMCID: PMC7580842
- DOI: 10.1080/20013078.2020.1769373
Triple knockdown of CDC37, HSP90-alpha and HSP90-beta diminishes extracellular vesicles-driven malignancy events and macrophage M2 polarization in oral cancer
Abstract
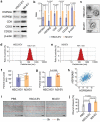
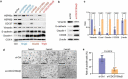
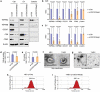
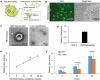
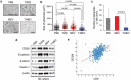
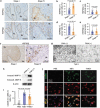
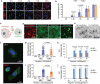
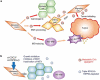
Evidence has been accumulating to indicate that extracellular vesicles (EVs), including exosomes, released by cancer cells can foster tumour progression. The molecular chaperones - CDC37, HSP90α and HSP90β play key roles in cancer progression including epithelial-mesenchymal transition (EMT), although their contribution to EVs-mediated cell-cell communication in tumour microenvironment has not been thoroughly examined. Here we show that triple depletion of the chaperone trio attenuates numerous cancer malignancy events exerted through EV release. Metastatic oral cancer-derived EVs (MEV) were enriched with HSP90α HSP90β and cancer-initiating cell marker CD326/EpCAM. Depletion of these chaperones individually induced compensatory increases in the other chaperones, whereas triple siRNA targeting of these molecules markedly diminished the levels of the chaperone trio and attenuated EMT. MEV were potent agents in initiating EMT in normal epithelial cells, a process that was attenuated by the triple chaperone depletion. The migration, invasion, and in vitro tumour initiation of oral cancer cells were significantly promoted by MEV, while triple depletion of CDC37/HSP90α/β reversed these MEV-driven malignancy events. In metastatic oral cancer patient-derived tumours, HSP90β was significantly accumulated in infiltrating tumour-associated macrophages (TAM) as compared to lower grade oral cancer cases. HSP90-enriched MEV-induced TAM polarization to an M2 phenotype, a transition known to support cancer progression, whereas the triple chaperone depletion attenuated this effect. Mechanistically, the triple chaperone depletion in metastatic oral cancer cells effectively reduced MEV transmission into macrophages. Hence, siRNA-mediated knockdown of the chaperone trio (CDC37/HSP90α/HSP90β) could potentially be a novel therapeutic strategy to attenuate several EV-driven malignancy events in the tumour microenvironment.
Abbreviations: CDC37: cell division control 37; EMT: epithelial-mesenchymal transmission; EV: extracellular vesicles; HNSCC: head and neck squamous cell carcinoma; HSP90: heat shock protein 90; TAM: tumour-associated macrophage.
Keywords: CDC37; Extracellular vesicles; HSP90; epithelial-mesenchymal transition; oral cancer; tetraspanin; tumour-associated macrophage.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group on behalf of The International Society for Extracellular Vesicles.
Figures

Similar articles
-
Cell Stress Induced Stressome Release Including Damaged Membrane Vesicles and Extracellular HSP90 by Prostate Cancer Cells.Cells. 2020 Mar 19;9(3):755. doi: 10.3390/cells9030755. Cells. 2020. PMID: 32204513 Free PMC article.
-
Multiple Targeting of HSP Isoforms to Challenge Isoform Specificity and Compensatory Expression.Methods Mol Biol. 2023;2693:141-161. doi: 10.1007/978-1-0716-3342-7_12. Methods Mol Biol. 2023. PMID: 37540433
-
Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted Hsp90alpha in tumour progression.Oncogene. 2017 Apr;36(15):2160-2171. doi: 10.1038/onc.2016.375. Epub 2016 Oct 10. Oncogene. 2017. PMID: 27721406 Free PMC article.
-
Targeting the Hsp90-Cdc37-client protein interaction to disrupt Hsp90 chaperone machinery.J Hematol Oncol. 2018 Apr 27;11(1):59. doi: 10.1186/s13045-018-0602-8. J Hematol Oncol. 2018. PMID: 29699578 Free PMC article. Review.
-
Targeting the HSP90-CDC37-kinase chaperone cycle: A promising therapeutic strategy for cancer.Med Res Rev. 2022 Jan;42(1):156-182. doi: 10.1002/med.21807. Epub 2021 Apr 12. Med Res Rev. 2022. PMID: 33846988 Review.
Cited by
-
Tumour-associated macrophage-derived DOCK7-enriched extracellular vesicles drive tumour metastasis in colorectal cancer via the RAC1/ABCA1 axis.Clin Transl Med. 2024 Feb;14(2):e1591. doi: 10.1002/ctm2.1591. Clin Transl Med. 2024. PMID: 38385857 Free PMC article.
-
HSP90 multi-functionality in cancer.Front Immunol. 2024 Aug 1;15:1436973. doi: 10.3389/fimmu.2024.1436973. eCollection 2024. Front Immunol. 2024. PMID: 39148727 Free PMC article. Review.
-
The Roles of Exosomes in the Diagnose, Development and Therapeutic Resistance of Oral Squamous Cell Carcinoma.Int J Mol Sci. 2023 Jan 19;24(3):1968. doi: 10.3390/ijms24031968. Int J Mol Sci. 2023. PMID: 36768288 Free PMC article. Review.
-
miR-4739 promotes epithelial-mesenchymal transition and angiogenesis in "driver gene-negative" non-small cell lung cancer via activating the Wnt/β-catenin signaling.Cell Oncol (Dordr). 2023 Dec;46(6):1821-1835. doi: 10.1007/s13402-023-00848-z. Epub 2023 Jul 27. Cell Oncol (Dordr). 2023. PMID: 37500965
-
Exosomes: Emerging Therapy Delivery Tools and Biomarkers for Kidney Diseases.Stem Cells Int. 2021 Aug 21;2021:7844455. doi: 10.1155/2021/7844455. eCollection 2021. Stem Cells Int. 2021. PMID: 34471412 Free PMC article. Review.
References
-
- Colombo M, Raposo G, Thery C.. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–21. - PubMed
-
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018. April;19(4):213–228. - PubMed
-
- Seyama M, Yoshida K, Yoshida K, et al. Outer membrane vesicles of Porphyromonas gingivalis attenuate insulin sensitivity by delivering gingipains to the liver. Biochim Biophys Acta Mol Basis Dis. 2020. February 20;1866(6):1–12. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

