Repeated radioembolization in advanced liver cancer
- PMID: 33145274
- PMCID: PMC7575953
- DOI: 10.21037/atm-20-2658
Repeated radioembolization in advanced liver cancer
Abstract
Background: To evaluate safety and clinical outcome of repeated transarterial 90Y (yttrium) radioembolization (TARE) in primary and metastatic liver cancer.
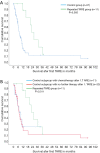
Methods: Between 2009 and 2018, n=288 patients underwent TARE for treatment of malignant liver disease in a tertiary care hospital. This retrospective single center study analyzed the safety and outcome of patients (n=11/288) undergoing repeated resin microsphere TARE. Included patients suffered from hepatocellular carcinoma (n=3), colorectal cancer (n=2), breast cancer (n=2), intrahepatic cholangiocarcinoma (n=3), and neuroendocrine carcinoma (n=1). All patients had shown either partial response (n=9) or stable disease (n=2) after first TARE. Lab parameters, response assessed by the Response Evaluation Criteria in Solid Tumors (mRECIST/RECIST) at 3 months and overall survival was analyzed. Additionally, patients with repeated TARE were compared to a matched control group (n=56) with single TARE therapy. Kaplan Meier analysis was performed to analyze survival.
Results: Patients after repeated TARE showed similar increase in lab parameters as compared to their first TARE. No case of radioembolization induced liver disease was observed. While n=5/11 patients showed a partial response and n=4/11 patients a stable disease after repeated TARE, only n=2/11 patients suffered from progressive disease. Median overall survival was 20.9±11.9 months for the repeated TARE group while it was 5.9±16.2 months for the control group.
Conclusions: Repeated 90Y TARE is safe and can be of benefit for patients yielding a comparable degree of local disease control compared to patients with singular TARE.
Keywords: 90Y yttrium; Repeated radioembolization; liver cancer.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2658). KR, MW, MK report receiving lectureship compensations and/or proctoring fees by SIRTeX Medical Europe, outside the submitted work. The other authors have no conflicts of interest to declare.
Figures
References
-
- Wasan HS, Gibbs P, Sharma NK, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol 2017;18:1159-71. 10.1016/S1470-2045(17)30457-6 - DOI - PMC - PubMed
-
- Gibbs P, Heinemann V, Sharma NK, et al. Effect of Primary Tumor Side on Survival Outcomes in Untreated Patients With Metastatic Colorectal Cancer When Selective Internal Radiation Therapy Is Added to Chemotherapy: Combined Analysis of Two Randomized Controlled Studies. Clin Colorectal Cancer 2018;17:e617-29. 10.1016/j.clcc.2018.06.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
