Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test
- PMID: 33145303
- PMCID: PMC7575971
- DOI: 10.21037/atm-20-5602
Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test
Abstract
Background: The characteristics, significance and potential cause of positive SARS-CoV-2 diagnoses in recovered coronavirus disease 2019 (COVID-19) patients post discharge (re-detectable positive, RP) remained elusive.
Methods: A total of 262 COVID-19 patients discharged from January 23 to February 25, 2020 were enrolled into this study. RP and non-RP (NRP) patients were grouped according to disease severity, and the characterization at re-admission was analyzed. SARS-CoV-2 RNA and plasma antibody levels were measured, and all patients were followed up for at least 14 days, with a cutoff date of March 10, 2020.
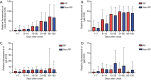
Results: A total of 14.5% of RP patients were detected. These patients were characterized as young and displayed mild and moderate conditions compared to NRP patients while no severe patients were RP. RP patients displayed fewer symptoms but similar plasma antibody levels during their hospitalization compared to NRP patients. Upon hospital readmission, these patients showed no obvious symptoms or disease progression. All 21 close contacts of RP patients were tested negative for viral RNA and showed no suspicious symptoms. Eighteen out of 24 of RNA-negative samples detected by the commercial kit were tested positive for viral RNA using a hyper-sensitive method, suggesting that these patients were potential carriers of the virus after recovery from COVID-19.
Conclusions: Our results indicated that young patients, with a mild diagnosis of COVID-19 are more likely to display RP status after discharge. These patients show no obvious symptoms or disease progression upon re-admission. More sensitive RNA detection methods are required to monitor these patients. Our findings provide information and evidence for the management of convalescent COVID-19 patients.
Keywords: Coronavirus; SARS-CoV-2; coronavirus disease 2019 (COVID-19).
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5602). The authors have no conflicts of interest to declare.
Figures


References
-
- Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.World Health Organization.
-
- WHO characterizes COVID-19 as a pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a... (accessed Mar 14, 2020).
-
- Wang M, Wu Q, Xu WZ, et al. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. medRxiv 2020. DOI: 10.1101/2020.02.12.20022327. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
