Management of hyponatremia associated with acute porphyria-proposal for the use of tolvaptan
- PMID: 33145317
- PMCID: PMC7575966
- DOI: 10.21037/atm-20-1529
Management of hyponatremia associated with acute porphyria-proposal for the use of tolvaptan
Abstract
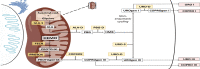
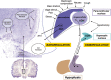
Hyponatremia is a common feature during the neurovisceral acute attacks which characterize hepatic porphyrias, as well as a sign of its severity. Therapeutic options for first-line acute attacks are intravenous administration of glucose and/or exogenous heme. The former treatment can aggravate hyponatremia by dilution and cause seizures; thus, the correction of hyponatremia must be carried out with extreme caution. This review summarizes recommendations for the management of hyponatremia during acute episodes of porphyria. Hyponatremia should be corrected slowly and seizures treated with medications in order to not exacerbate motor and sensory axonal neuropathy. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is considered a frequent cause of hyponatremia in acute porphyrias and must be identified as a symptom of an acute porphyria attack. Tolvaptan produces aquaresis and is considered a safe drug in porphyria. However, its use has only been reported in isolated cases during a porphyria attack. The convenience and usefulness of this drug in acute porphyria are discussed.
Keywords: Acute hepatic porphyrias; hyponatremia; management; syndrome of inappropriate antidiuretic hormone secretion (SIADH); tolvaptan.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure from (available at http://dx.doi.org/10.21037/atm-20-1529). The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Rapidity of Correction of Hyponatremia Due to Syndrome of Inappropriate Secretion of Antidiuretic Hormone Following Tolvaptan.Am J Kidney Dis. 2018 Jun;71(6):772-782. doi: 10.1053/j.ajkd.2017.12.002. Epub 2018 Feb 23. Am J Kidney Dis. 2018. PMID: 29478867 Free PMC article.
-
SIADH-related hyponatremia in hospital day care units: clinical experience and management with tolvaptan.Support Care Cancer. 2016 Jan;24(1):499-507. doi: 10.1007/s00520-015-2948-6. Epub 2015 Oct 2. Support Care Cancer. 2016. PMID: 26431960 Free PMC article.
-
[The treament of hyponatremia secundary to the syndrome of inappropriate antidiuretic hormone secretion].Med Clin (Barc). 2013 Dec 7;141(11):507.e1-507.e10. doi: 10.1016/j.medcli.2013.09.002. Epub 2013 Oct 26. Med Clin (Barc). 2013. PMID: 24169317 Spanish.
-
Diagnosing and Treating the Syndrome of Inappropriate Antidiuretic Hormone Secretion.Am J Med. 2016 May;129(5):537.e9-537.e23. doi: 10.1016/j.amjmed.2015.11.005. Epub 2015 Nov 14. Am J Med. 2016. PMID: 26584969
-
Acute Porphyrias.J Emerg Med. 2015 Sep;49(3):305-12. doi: 10.1016/j.jemermed.2015.04.034. Epub 2015 Jul 7. J Emerg Med. 2015. PMID: 26159905 Review.
Cited by
-
Cutting-Edge Therapies and Novel Strategies for Acute Intermittent Porphyria: Step-by-Step towards the Solution.Biomedicines. 2022 Mar 11;10(3):648. doi: 10.3390/biomedicines10030648. Biomedicines. 2022. PMID: 35327450 Free PMC article. Review.
-
Therapy Follows Diagnosis: Old and New Approaches for the Treatment of Acute Porphyrias, What We Know and What We Should Know.Diagnostics (Basel). 2022 Jul 3;12(7):1618. doi: 10.3390/diagnostics12071618. Diagnostics (Basel). 2022. PMID: 35885523 Free PMC article. Review.
-
Red urine and a red herring - diagnosing rare diseases in the light of the COVID-19 pandemic.Z Gastroenterol. 2022 Sep;60(9):1326-1331. doi: 10.1055/a-1659-4481. Epub 2021 Nov 12. Z Gastroenterol. 2022. PMID: 34768287 Free PMC article.
-
A 38-year-Old Woman With Flaccid Tetraparesis after Presenting With Abdominal Pain.Neurohospitalist. 2025 Apr;15(2):172-175. doi: 10.1177/19418744241281000. Epub 2024 Sep 2. Neurohospitalist. 2025. PMID: 39555118 Free PMC article.
-
A Comprehensive Review of Electrolyte Imbalances and Their Applied Aspects in Dermatology.Cureus. 2025 Mar 28;17(3):e81353. doi: 10.7759/cureus.81353. eCollection 2025 Mar. Cureus. 2025. PMID: 40291321 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
