Mesenteric Lymph Duct Drainage Attenuates Lung Inflammatory Injury and Inhibits Endothelial Cell Apoptosis in Septic Rats
- PMID: 33145344
- PMCID: PMC7596461
- DOI: 10.1155/2020/3049302
Mesenteric Lymph Duct Drainage Attenuates Lung Inflammatory Injury and Inhibits Endothelial Cell Apoptosis in Septic Rats
Abstract
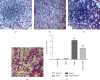
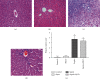
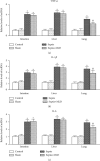
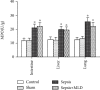
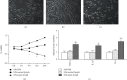
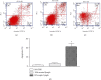
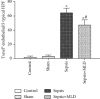
The present study was to investigate the effect of mesenteric lymph duct drainage on lung inflammatory response, histological alteration, and endothelial cell apoptosis in septic rats. Animals were randomly assigned into four groups: control, sham surgery, sepsis, and sepsis plus mesenteric lymph drainage. We used the colon ascendens stent peritonitis (CASP) procedure to induce the septic model in rats, and mesenteric lymph drainage was performed with a polyethylene (PE) catheter inserted into mesenteric lymphatic. The animals were sacrificed at the end of CASP in 6 h. The mRNA expression levels of inflammatory mediators were measured by qPCR, and the histologic damage were evaluated by the pathological score method. It was found that mesenteric lymph drainage significantly reduced the expression of TNF-α, IL-1β, and IL-6 mRNA in the lung. Pulmonary interstitial edema and infiltration of inflammatory cells were alleviated by mesenteric lymph drainage. Moreover, increased mRNA levels of TNF-α, IL-1β, IL-6 mRNA, and apoptotic rate were observed in PMVECs treated with septic lymph. These results indicate that mesenteric lymph duct drainage significantly attenuated lung inflammatory injury by decreasing the expression of pivotal inflammatory mediators and inhibiting endothelial apoptosis to preserve the pulmonary barrier function in septic rats.
Copyright © 2020 Yongjun Liu et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
[Protecting effect of mesenteric lymph duct ligation on vital organs of rats with hemorrhagic shock].Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009 May;21(5):274-7. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009. PMID: 19439113 Chinese.
-
Intravenous infusion of mesenteric lymph from severe intraperitoneal infection rats causes lung injury in healthy rats.World J Gastroenterol. 2014 Apr 28;20(16):4771-7. doi: 10.3748/wjg.v20.i16.4771. World J Gastroenterol. 2014. PMID: 24782631 Free PMC article.
-
Biliary tract external drainage improves inflammatory mediators and pathomorphology of the intestine, liver, and lung in septic rats.J Trauma Acute Care Surg. 2018 Sep;85(3):580-587. doi: 10.1097/TA.0000000000001979. J Trauma Acute Care Surg. 2018. PMID: 29847538
-
The Gut-Lung Axis in Systemic Inflammation. Role of Mesenteric Lymph as a Conduit.Am J Respir Cell Mol Biol. 2021 Jan;64(1):19-28. doi: 10.1165/rcmb.2020-0196TR. Am J Respir Cell Mol Biol. 2021. PMID: 32877613 Free PMC article. Review.
-
Therapeutic thoracic duct drainage: A systematic review of the Eastern European experience and future potential.Lymphology. 2022;55(3):86-109. Lymphology. 2022. PMID: 36446397
Cited by
-
Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis.Cells. 2021 Nov 25;10(12):3305. doi: 10.3390/cells10123305. Cells. 2021. PMID: 34943813 Free PMC article.
-
The Gut-Lung Axis During Ethanol Exposure and a Pseudomonas aeruginosa Bacterial Challenge.Biomedicines. 2024 Dec 3;12(12):2757. doi: 10.3390/biomedicines12122757. Biomedicines. 2024. PMID: 39767664 Free PMC article.
-
Intestinal Microbiota - An Unmissable Bridge to Severe Acute Pancreatitis-Associated Acute Lung Injury.Front Immunol. 2022 Jun 14;13:913178. doi: 10.3389/fimmu.2022.913178. eCollection 2022. Front Immunol. 2022. PMID: 35774796 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

