Rapamycin-Induced Autophagy Promotes the Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells in the Temporomandibular Joint in Response to IL-1 β
- PMID: 33145347
- PMCID: PMC7599423
- DOI: 10.1155/2020/4035306
Rapamycin-Induced Autophagy Promotes the Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells in the Temporomandibular Joint in Response to IL-1 β
Abstract
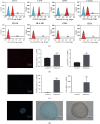
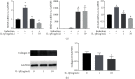
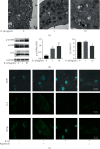
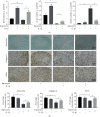
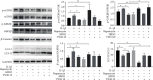
Cartilage defects in temporomandibular disorders (TMD) lead to chronic pain and seldom heal. Synovium-derived mesenchymal stem cells (SMSCs) exhibit superior chondrogenesis and have become promising seed cells for cartilage tissue engineering. However, local inflammatory conditions that affect the repair of articular cartilage by SMSCs present a challenge, and the specific mechanism through which the function remains unclear. Thus, it is important to explore the chondrogenesis of SMSCs under inflammatory conditions of TMD such that they can be used more effectively in clinical treatment. In this study, we obtained SMSCs from TMD patients with severe cartilage injuries. In response to stimulation with IL-1β, which is well known as one of the most prevalent cytokines in TMD, MMP13 expression increased, while that of SOX9, aggrecan, and collagen II decreased during chondrogenic differentiation. At the same time, IL-1β upregulated the expression of mTOR and decreased the ratio of LC3-II/LC3-I and the formation of autophagosomes. Further study revealed that rapamycin pretreatment promoted the migration of SMSCs and the expression of chondrogenesis-related markers in the presence of IL-1β by inducing autophagy. 3-Benzyl-5-((2-nitrophenoxy)methyl)-dihydrofuran-2(3H)-one (3BDO), a new activator of mTOR, inhibited autophagy and increased the expression of p-GSK3βser9 and β-catenin, simulating the effect of IL-1β stimulation. Furthermore, rapamycin reduced the expression of mTOR, whereas the promotion of LC3-II/LC3-I was blocked by the GSK3β inhibitor TWS119. Taken together, these results indicate that rapamycin enhances the chondrogenesis of SMSCs by inducing autophagy, and GSK3β may be an important regulator in the process of rapamycin-induced autophagy. Thus, inducing autophagy may be a useful approach in the chondrogenic differentiation of SMSCs in the inflammatory microenvironment and may represent a novel TMD treatment.
Copyright © 2020 Wenjing Liu et al.
Conflict of interest statement
The authors declare no conflicts of interest in relation to this paper.
Figures

References
-
- Wang Y. L., Liu J., Wan L., Ruan L. P., Ye W. F. Effect of Xinfeng capsule on Beclin 1/PI3K-AKT-mTOR of adjuvant arthritis rats. Chinese Journal of Integrated Traditional and Western Medicine. 2017;37(4):464–469. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

