Engineering and In Vitro Selection of a Novel AAV3B Variant with High Hepatocyte Tropism and Reduced Seroreactivity
- PMID: 33145371
- PMCID: PMC7591349
- DOI: 10.1016/j.omtm.2020.09.019
Engineering and In Vitro Selection of a Novel AAV3B Variant with High Hepatocyte Tropism and Reduced Seroreactivity
Abstract
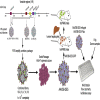
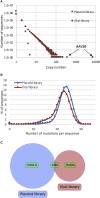
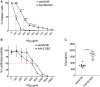
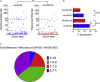
Limitations to successful gene therapy with adeno-associated virus (AAV) can comprise pre-existing neutralizing antibodies to the vector capsid that can block cellular entry, or inefficient transduction of target cells that can lead to sub-optimal expression of the therapeutic transgene. Recombinant serotype 3 AAV (AAV3) is an emerging candidate for liver-directed gene therapy. In this study, we integrated rational design by using a combinatorial library derived from AAV3B capsids with directed evolution by in vitro selection for liver-targeted AAV variants. The AAV3B-DE5 variant described herein was undetectable in the original viral library but gained a selective advantage upon in vitro passaging in human hepatocarcinoma spheroid cultures. AAV3B-DE5 contains 24 capsid amino acid substitutions compared with AAV3B, distributed among all five variable regions, with strong selective pressure on VR-IV, VR-V, and VR-VII. In vivo, AAV3B-DE5 demonstrated improved human hepatocyte tropism in a liver chimeric mouse model. Importantly, this variant exhibited reduced seroreactivity to human intravenous immunoglobulin (i.v. Ig), as well as individual serum samples from 100 healthy human donors. Therefore, molecular evolution using a combinatorial library platform generated a viral capsid with high hepatocyte tropism and enhanced evasion of pre-existing AAV neutralizing antibodies.
Keywords: AAV3; capsid library; directed evolution; gene therapy; hepatocyte; liver tropism; neutralizing antibody; seroprevalence.
© 2020 The Authors.
Figures





References
-
- Anguela X.M., High K.A. Entering the modern era of gene therapy. Annu. Rev. Med. 2019;70:273–288. - PubMed
-
- Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. - PubMed
-
- Bennett J., Wellman J., Marshall K.A., McCague S., Ashtari M., DiStefano-Pappas J., Elci O.U., Chung D.C., Sun J., Wright J.F. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–672. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

