Metal@Zeolite Hybrid Materials for Catalysis
- PMID: 33145408
- PMCID: PMC7596864
- DOI: 10.1021/acscentsci.0c01130
Metal@Zeolite Hybrid Materials for Catalysis
Abstract
The fixation of metal nanoparticles into zeolite crystals has emerged as a new series of heterogeneous catalysts, giving performances that steadily outperform the generally supported catalysts in many important reactions. In this outlook, we define different noble metal-in-zeolite structures (metal@zeolite) according to the size of the nanoparticles and their relative location to the micropores. The metal species within the micropores and those larger than the micropores are denoted as encapsulated and fixed structures, respectively. The development in the strategies for the construction of metal@zeolite hybrid materials is briefly summarized in this work, where the rational preparation and improved thermal stability of the metal nanostructures are particularly mentioned. More importantly, these metal@zeolite hybrid materials as catalysts exhibit excellent shape selectivity. Finally, we review the current challenges and future perspectives for these metal@zeolite catalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
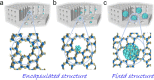




References
-
- van Deelen T. W.; Mejía C. H.; de Jong K. P. Control of Metal-Support Interactions in Heterogeneous Catalysts to Enhance Activity and Selectivity. Nat. Catal. 2019, 2, 955–970. 10.1038/s41929-019-0364-x. - DOI
-
- Otor H. O.; Steiner J. B.; García-Sancho C.; Alba-Rubio A. C. Encapsulation Methods for Control of Catalyst Deactivation: A Review. ACS Catal. 2020, 10, 7630–7656. 10.1021/acscatal.0c01569. - DOI
-
- Wang A.; Li J.; Zhang T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81. 10.1038/s41570-018-0010-1. - DOI
-
- Parastaev A.; Muravev V.; Osta E. H.; van Hoof A. J. F.; Kimpel T. F.; Kosinov N.; Hensen E. J. M. Boosting CO2 Hydrogenation via Size-Dependent Metal-Support Interactions in Cobalt/Ceria-Based Catalysts. Nat. Catal. 2020, 3, 526–533. 10.1038/s41929-020-0459-4. - DOI
Publication types
LinkOut - more resources
Full Text Sources

