Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity
- PMID: 33145416
- PMCID: PMC7596866
- DOI: 10.1021/acscentsci.0c00732
Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity
Abstract
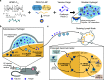
Vaccines aim to elicit a robust, yet targeted, immune response. Failure of a vaccine to elicit such a response arises in part from inappropriate temporal control over antigen and adjuvant presentation to the immune system. In this work, we sought to exploit the immune system's natural response to extended pathogen exposure during infection by designing an easily administered slow-delivery vaccine platform. We utilized an injectable and self-healing polymer-nanoparticle (PNP) hydrogel platform to prolong the codelivery of vaccine components to the immune system. We demonstrated that these hydrogels exhibit unique delivery characteristics, whereby physicochemically distinct compounds (such as antigen and adjuvant) could be codelivered over the course of weeks. When administered in mice, hydrogel-based sustained vaccine exposure enhanced the magnitude, duration, and quality of the humoral immune response compared to standard PBS bolus administration of the same model vaccine. We report that the creation of a local inflammatory niche within the hydrogel, coupled with sustained exposure of vaccine cargo, enhanced the magnitude and duration of germinal center responses in the lymph nodes. This strengthened germinal center response promoted greater antibody affinity maturation, resulting in a more than 1000-fold increase in antigen-specific antibody affinity in comparison to bolus immunization. In summary, this work introduces a simple and effective vaccine delivery platform that increases the potency and durability of subunit vaccines.
Conflict of interest statement
The authors declare the following competing financial interest(s): G.A.R., E.C.G., M.M.D., and E.A.A. are inventors on a patent describing the technology reported in this manuscript.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

