Taurine mitigates cirrhosis-associated heart injury through mitochondrial-dependent and antioxidative mechanisms
- PMID: 33145427
- PMCID: PMC7592093
- DOI: 10.5114/ceh.2020.99513
Taurine mitigates cirrhosis-associated heart injury through mitochondrial-dependent and antioxidative mechanisms
Abstract
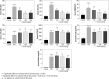
Cirrhosis-induced heart injury and cardiomyopathy is a serious consequence of this disease. It has been shown that bile duct ligated (BDL) animals could serve as an appropriate experimental model to investigate heart tissue injury in cirrhosis. The accumulation of cytotoxic chemicals (e.g., bile acids) could also adversely affect the heart tissue. Oxidative stress and mitochondrial impairment are the most prominent mechanisms of bile acid cytotoxicity. Taurine (Tau) is the most abundant non-protein amino acid in the human body. The cardioprotective effects of this amino acid have repeatedly been investigated. In the current study, it was examined whether mitochondrial dysfunction and oxidative stress are involved in the pathogenesis of cirrhosis-induced heart injury. Rats underwent BDL surgery. BDL animals received Tau (50, 100, and 500 mg/kg, i.p.) for 42 consecutive days. A significant increase in oxidative stress biomarkers was detected in the heart tissue of BDL animals. Moreover, it was found that heart tissue mitochondrial indices of functionality were deteriorated in the BDL group. Tau treatment significantly decreased oxidative stress and improved mitochondrial function in the heart tissue of cirrhotic animals. These data provide clues for the involvement of mitochondrial impairment and oxidative stress in the pathogenesis of heart injury in BDL rats. On the other hand, Tau supplementation could serve as an effective ancillary treatment against BDL-associated heart injury. Mitochondrial regulating and antioxidative properties of Tau might play a fundamental role in its mechanism of protective effects in the heart tissue of BDL animals.
Keywords: amino acid; bioenergetics; cardiomyopathy; cirrhosis; heart failure; mitochondrial impairment.
Copyright © 2020 Clinical and Experimental Hepatology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Milani A, Zaccaria R, Bombardieri G, et al. . Cirrhotic cardiomyopathy. Dig Liver Dis 2007; 39: 507-515. - PubMed
-
- Zardi EM, Abbate A, Zardi DM, et al. . Cirrhotic cardiomyopathy. J Am Coll Cardiol 2010; 56: 539-549. - PubMed
-
- Wiese S, Hove JD, Bendtsen F, Møller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 2014; 11: 177-186. - PubMed
-
- Møller S, Hove JD, Dixen U, Bendtsen F. New insights into cirrhotic cardiomyopathy. Int J Cardiol 2013; 167: 1101-1108. - PubMed
-
- Gaskari SA, Honar H, Lee SS. Therapy insight: Cirrhotic cardiomyopathy. Gastroenterol Hepatol (N Y) 2006; 3: 329-337. - PubMed
LinkOut - more resources
Full Text Sources
