A randomised controlled feasibility trial of E-health application supported care vs usual care after exacerbation of COPD: the RESCUE trial
- PMID: 33145441
- PMCID: PMC7603326
- DOI: 10.1038/s41746-020-00347-7
A randomised controlled feasibility trial of E-health application supported care vs usual care after exacerbation of COPD: the RESCUE trial
Erratum in
-
Author Correction: A randomised controlled feasibility trial of E-health application supported care vs usual care after exacerbation of COPD: the RESCUE trial.NPJ Digit Med. 2020 Nov 12;3(1):150. doi: 10.1038/s41746-020-00360-w. NPJ Digit Med. 2020. PMID: 33299051 Free PMC article. No abstract available.
Abstract
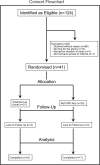
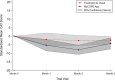
Exacerbations of COPD are one of the commonest causes of admission and readmission to hospital. The role of digital interventions to support self-management in improving outcomes is uncertain. We conducted an open, randomised controlled trial of a digital health platform application (app) in 41 COPD patients recruited following hospital admission with an acute exacerbation. Subjects were randomised to either receive usual care, including a written self-management plan (n = 21), or the myCOPD app (n = 20) for 90 days. The primary efficacy outcome was recovery rate of symptoms measured by COPD assessment test (CAT) score. Exacerbations, readmission, inhaler technique quality of life and patient activation (PAM) scores were also captured by a blinded team. The app was acceptable in this care setting and was used by 17 of the 20 patients with sustained use over the study period. The treatment effect on the CAT score was 4.49 (95% CI: -8.41, -0.58) points lower in the myCOPD arm. Patients' inhaler technique improved in the digital intervention arm (101 improving to 20 critical errors) compared to usual care (100 to 72 critical errors). Exacerbations tended to be less frequent in the digital arm compared to usual care; 34 vs 18 events. Hospital readmissions risk was numerically lower in the digital intervention arm: OR for readmission 0.383 (95% CI: 0.074, 1.987; n = 35). In this feasibility study of the digital self-management platform myCOPD, the app has proven acceptable to patients to use and use has improved exacerbation recovery rates, with strong signals of lower re-exacerbation and readmission rates over 90 days. myCOPD reduced the number of critical errors in inhaler technique compared to usual care with written self-management. This provides a strong basis for further exploration of the use of app interventions in the context of recently hospitalised patients with COPD and informs the potential design of a large multi-centre trial.
Keywords: Disease prevention; Respiratory tract diseases.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsT.W. is the co-founder, shareholder and director, S.B. is the co-founder, shareholder and director M.N. and A.B are employees of Mymhealth Limited. T.W., S.B. and M.N. have received personal fees during the conduct of the study. B.G. and T.B. received grants from Mymhealth Limited. Outside the submitted work, T.B. has received grants from GSK and personal fees from AstraZeneca, GSK, Teva, Napp Pharmaceuticals and Novartis as well as non-financial support from GSK, Teva and Novartis. Outside the submitted work, T.W. has received grants from GSK, AstraZeneca and Synairgen and has received personal fees from AstraZeneca, Synairgen and BI. A.J.C., J.W., T.J., D.N., A.W., D.C., M.J., J.E. and V.C. have nothing to disclose.
Figures


References
-
- Snell N, Strachan D, Hubbard R, Gibson J, Gruffydd-Jones K, et al. S32 Epidemiology of chronic obstructive pulmonary disease (COPD) in the uk: findings from the british lung foundation’s ‘respiratory health of the nation’ project. Thorax. 2016;71:A20–A20.
-
- BLF. Out in the Cold Lung Disease The Hidden Driver of NHS Winter Pressures (British Lung Foundation, London, 2017).
-
- Department of Health. Long Term Conditions Compendium of Information. 3rd edn (Department of Health, 2012).
-
- Williams NP, Coombs NA, Johnson MJ, Josephs LK, Rigge LA, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:313–322. doi: 10.2147/COPD.S121389. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

