Interaction of amyloid beta with humanin and acetylcholinesterase is modulated by ATP
- PMID: 33145964
- PMCID: PMC7714071
- DOI: 10.1002/2211-5463.13023
Interaction of amyloid beta with humanin and acetylcholinesterase is modulated by ATP
Abstract
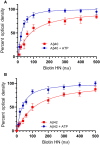
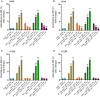
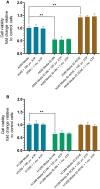
Humanin (HN) is known to bind amyloid beta (Aβ)-inducing cytoprotective effects, while binding of acetylcholinesterase (AChE) to Aβ increases its aggregation and cytotoxicity. Previously, we showed that binding of HN to Aβ blocks aggregation induced by AChE and that HN decreases but does not abolish Aβ-AChE interactions in A549 cell media. Here, we set out to shed light on factors that modulate the interactions of Aβ with HN and AChE. We found that binding of either HN or AChE to Aβ is not affected by heparan sulfate, while ATP, thought to reduce misfolding of Aβ, weakened interactions between AChE and Aβ but strengthened those between Aβ and HN. Using media from either A549 or H1299 lung cancer cells, we observed that more HN was bound to Aβ upon addition of ATP, while levels of AChE in a complex with Aβ were decreased by ATP addition to A549 cell media. Exogenous addition of ATP to either A549 or H1299 cell media increased interactions of endogenous HN with Aβ to a comparable extent despite differences in AChE expression in the two cell lines, and this was correlated with decreased binding of exogenously added HN to Aβ. Treatment with exogenous ATP had no effect on cell viability under all conditions examined. Exogenously added ATP did not affect viability of cells treated with AChE-immunodepleted media, and there was no apparent protection against the cytotoxicity resulting from immunodepletion of HN. Moreover, exogenously added ATP had no effect on the relative abundance of oligomer versus total Aβ in either cell line.
Keywords: acetylcholinesterase; amyloid-beta; humanin; kinetics; lung cancer; peptide interaction.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures






Similar articles
-
Humanin Blocks the Aggregation of Amyloid-β Induced by Acetylcholinesterase, an Effect Abolished in the Presence of IGFBP-3.Biochemistry. 2020 Jun 2;59(21):1981-2002. doi: 10.1021/acs.biochem.0c00274. Epub 2020 May 20. Biochemistry. 2020. PMID: 32383868 Free PMC article.
-
Using Small Peptide Segments of Amyloid-β and Humanin to Examine their Physical Interactions.Protein Pept Lett. 2019;26(7):502-511. doi: 10.2174/0929866526666190405122117. Protein Pept Lett. 2019. PMID: 30950343
-
Humanin Specifically Interacts with Amyloid-β Oligomers and Counteracts Their in vivo Toxicity.J Alzheimers Dis. 2017;57(3):857-871. doi: 10.3233/JAD-160951. J Alzheimers Dis. 2017. PMID: 28282805
-
Acetylcholinesterase-amyloid-beta-peptide interaction: effect of Congo Red and the role of the Wnt pathway.Curr Alzheimer Res. 2005 Jul;2(3):301-6. doi: 10.2174/1567205054367928. Curr Alzheimer Res. 2005. PMID: 15974895 Review.
-
Acetylcholinesterase (AChE)--amyloid-beta-peptide complexes in Alzheimer's disease. the Wnt signaling pathway.Curr Alzheimer Res. 2004 Nov;1(4):249-54. doi: 10.2174/1567205043332063. Curr Alzheimer Res. 2004. PMID: 15975054 Review.
Cited by
-
Regulation of Soluble E-Cadherin Signaling in Non-Small-Cell Lung Cancer Cells by Nicotine, BDNF, and β-Adrenergic Receptor Ligands.Biomedicines. 2023 Sep 18;11(9):2555. doi: 10.3390/biomedicines11092555. Biomedicines. 2023. PMID: 37760996 Free PMC article.
-
Regulation of the Soluble Amyloid Precursor Protein α (sAPPα) Levels by Acetylcholinesterase and Brain-Derived Neurotrophic Factor in Lung Cancer Cell Media.Int J Mol Sci. 2022 Sep 15;23(18):10746. doi: 10.3390/ijms231810746. Int J Mol Sci. 2022. PMID: 36142659 Free PMC article.
-
Regulation of Cisplatin Resistance in Lung Cancer Cells by Nicotine, BDNF, and a β-Adrenergic Receptor Blocker.Int J Mol Sci. 2022 Oct 24;23(21):12829. doi: 10.3390/ijms232112829. Int J Mol Sci. 2022. PMID: 36361620 Free PMC article.
-
Multitargeted Molecular Docking and Dynamic Simulation Studies of Bioactive Compounds from Rosmarinus officinalis against Alzheimer's Disease.Molecules. 2022 Oct 25;27(21):7241. doi: 10.3390/molecules27217241. Molecules. 2022. PMID: 36364071 Free PMC article.
-
Phosphorylation of IGFBP-3 by Casein Kinase 2 Blocks Its Interaction with Hyaluronan, Enabling HA-CD44 Signaling Leading to Increased NSCLC Cell Survival and Cisplatin Resistance.Cells. 2023 Jan 25;12(3):405. doi: 10.3390/cells12030405. Cells. 2023. PMID: 36766747 Free PMC article.
References
-
- Qian Y, Wang X, Li Y, Cao Y and Chen X (2016) Extracellular ATP a new player in cancer metabolism: NSCLC cells internalize ATP in vitro and in vivo using multiple endocytic mechanisms. Mol Cancer Res 14, 1087–1096. - PubMed
-
- Schwiebert EM and Zsembery A (2003) Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615, 7–32. - PubMed
-
- Villegas S (2015) Alzheimer’s disease: new therapeutic strategies. Med Clin 145, 76–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

