Prefoldin subunit 6 of Plasmodium falciparum binds merozoite surface protein-1
- PMID: 33145997
- PMCID: PMC9063436
- DOI: 10.1002/2211-5463.13022
Prefoldin subunit 6 of Plasmodium falciparum binds merozoite surface protein-1
Abstract
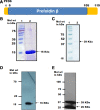
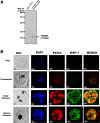
Malaria is a human disease caused by eukaryotic protozoan parasites of the Plasmodium genus. Plasmodium falciparum (Pf) causes the most lethal form of human malaria and is responsible for widespread mortality worldwide. Prefoldin is a heterohexameric molecular complex that binds and delivers unfolded proteins to chaperonin for correct folding. The prefoldin PFD6 is predicted to interact with merozoite surface protein-1 (MSP-1), a protein well known to play a pivotal role in erythrocyte binding and invasion by Plasmodium merozoites. We previously found that the P. falciparum (Pf) genome contains six prefoldin genes and a prefoldin-like gene whose molecular functions are unidentified. Here, we analyzed the expression of PfPFD-6 during the asexual blood stages of the parasite and investigated its interacting partners. PfPFD-6 was found to be significantly expressed at the trophozoite and schizont stages. Pull-down assays suggest PfPFD-6 interacts with MSP-1. In silico analysis suggested critical residues involved in the PfPFD-6-MSP-1 interaction. Our data suggest PfPFD-6 may play a role in stabilizing or trafficking MSP-1.
Keywords: Plasmodium falciparum; chaperone; malaria; merozoite surface protein-1; prefoldin.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

