Nicotine exhausts CD8+ T cells against tumor cells through increasing miR-629-5p to repress IL2RB-mediated granzyme B expression
- PMID: 33146402
- PMCID: PMC10991426
- DOI: 10.1007/s00262-020-02770-x
Nicotine exhausts CD8+ T cells against tumor cells through increasing miR-629-5p to repress IL2RB-mediated granzyme B expression
Abstract
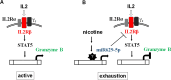
The mechanism exhausting CD8+ T cells is not completely clear against tumors. Literature has demonstrated that cigarette smoking disables the immunological activity, so we propose nicotine is able to exhaust CD8+ T cells. The CD8+ T cells from healthy volunteers with and without cigarette smoking and the capacity of CD8+ T cells against tumor cells were investigated. RNAseq was used to investigate the gene profiling expression in CD8+ T cells. Meanwhile, small RNAseq was also used to search novel microRNAs involved in the exhaustion of CD8+ T cells. The effect of nicotine exhausting CD8+ T cells was investigated in vitro and in the humanized tumor xenografts in vivo. We found that CD8+ T cells were able to reduce cell viability in lung cancer HCC827 and A549 cells, that secreted granzyme B, but CD8+ T cells from the healthy cigarette smokers lost anti-HCC827 effect. Moreover, nicotine suppressed the anti-HCC827 effect of CD8+ T cells. RNAseq revealed lower levels of IL2RB and GZMB in the exhausted CD8+ T cells. We identified that miR-629-5p was increased by nicotine, that targeted IL2RB. Transfection of miR-629-5p mimic reduced IL2RB and GZMB levels. We further validated that nicotine reduced granzyme B levels using a nuclear imaging technique, and demonstrated that nicotine exhausted peripheral blood mononuclear cells against HCC827 growth in the humanized tumor xenografts. This study demonstrated that nicotine exhausted CD8+ T cells against HCC827 cells through increasing miR-629-5p to suppress IL2RB.
Keywords: CD8+ T cells; Granzyme B; IL2RB; Nicotine.
Conflict of interest statement
We declare that no conflict of interest for this article.
Figures





Similar articles
-
Irradiation Suppresses IFNγ-Mediated PD-L1 and MCL1 Expression in EGFR-Positive Lung Cancer to Augment CD8+ T Cells Cytotoxicity.Cells. 2021 Sep 23;10(10):2515. doi: 10.3390/cells10102515. Cells. 2021. PMID: 34685495 Free PMC article.
-
Nicotine Changes the microRNA Profile to Regulate the FOXO Memory Program of CD8+ T Cells in Rheumatoid Arthritis.Front Immunol. 2020 Jul 14;11:1474. doi: 10.3389/fimmu.2020.01474. eCollection 2020. Front Immunol. 2020. PMID: 32765511 Free PMC article.
-
Downregulation of miR-497-5p Improves Sepsis-Induced Acute Lung Injury by Targeting IL2RB.Biomed Res Int. 2021 Apr 12;2021:6624702. doi: 10.1155/2021/6624702. eCollection 2021. Biomed Res Int. 2021. PMID: 33954185 Free PMC article.
-
Memory-promoting function of miR-379-5p attenuates CD8+ T cell exhaustion by targeting immune checkpoints.J Immunother Cancer. 2025 Apr 12;13(4):e010363. doi: 10.1136/jitc-2024-010363. J Immunother Cancer. 2025. PMID: 40221151 Free PMC article.
-
Genome-wide profiling of micro-RNA expression in gefitinib-resistant human lung adenocarcinoma using microarray for the identification of miR-149-5p modulation.Tumour Biol. 2017 Mar;39(3):1010428317691659. doi: 10.1177/1010428317691659. Tumour Biol. 2017. PMID: 28345454
Cited by
-
Irradiation Suppresses IFNγ-Mediated PD-L1 and MCL1 Expression in EGFR-Positive Lung Cancer to Augment CD8+ T Cells Cytotoxicity.Cells. 2021 Sep 23;10(10):2515. doi: 10.3390/cells10102515. Cells. 2021. PMID: 34685495 Free PMC article.
-
The role of microRNAs in nicotine signaling.EXCLI J. 2023 May 19;22:433-450. doi: 10.17179/excli2023-6096. eCollection 2023. EXCLI J. 2023. PMID: 37346805 Free PMC article. Review.
-
Cell signaling and epigenetic regulation of nicotine-induced carcinogenesis.Environ Pollut. 2024 Mar 15;345:123426. doi: 10.1016/j.envpol.2024.123426. Epub 2024 Jan 29. Environ Pollut. 2024. PMID: 38295934 Free PMC article. Review.
-
The effect of smoking on tumor immunoediting: Friend or foe?Tob Induc Dis. 2024 Jun 17;22. doi: 10.18332/tid/189302. eCollection 2024. Tob Induc Dis. 2024. PMID: 38887597 Free PMC article. Review.
-
The feasibility of granzyme B levels using an amperometric immunosensor for lung cancer detection.Ann Transl Med. 2022 Jun;10(11):622. doi: 10.21037/atm-22-470. Ann Transl Med. 2022. PMID: 35813317 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

