Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial
- PMID: 33146667
- PMCID: PMC7643046
- DOI: 10.1001/jamapsychiatry.2020.3285
Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial
Erratum in
-
Errors in Response Rate, Effect Sizes, and Confidence Intervals.JAMA Psychiatry. 2021 May;78(5):569. doi: 10.1001/jamapsychiatry.2020.4714. Epub 2021 Feb 10. JAMA Psychiatry. 2021. PMID: 33566065 Free PMC article. No abstract available.
Abstract
Importance: Major depressive disorder (MDD) is a substantial public health burden, but current treatments have limited effectiveness and adherence. Recent evidence suggests that 1 or 2 administrations of psilocybin with psychological support produces antidepressant effects in patients with cancer and in those with treatment-resistant depression.
Objective: To investigate the effect of psilocybin therapy in patients with MDD.
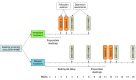
Design, setting, and participants: This randomized, waiting list-controlled clinical trial was conducted at the Center for Psychedelic and Consciousness Research at Johns Hopkins Bayview Medical Center in Baltimore, Maryland. Adults aged 21 to 75 years with an MDD diagnosis, not currently using antidepressant medications, and without histories of psychotic disorder, serious suicide attempt, or hospitalization were eligible to participate. Enrollment occurred between August 2017 and April 2019, and the 4-week primary outcome assessments were completed in July 2019. A total of 27 participants were randomized to an immediate treatment condition group (n = 15) or delayed treatment condition group (waiting list control condition; n = 12). Data analysis was conducted from July 1, 2019, to July 31, 2020, and included participants who completed the intervention (evaluable population).
Interventions: Two psilocybin sessions (session 1: 20 mg/70 kg; session 2: 30 mg/70 kg) were given (administered in opaque gelatin capsules with approximately 100 mL of water) in the context of supportive psychotherapy (approximately 11 hours). Participants were randomized to begin treatment immediately or after an 8-week delay.
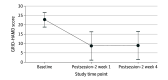
Main outcomes and measures: The primary outcome, depression severity was assessed with the GRID-Hamilton Depression Rating Scale (GRID-HAMD) scores at baseline (score of ≥17 required for enrollment) and weeks 5 and 8 after enrollment for the delayed treatment group, which corresponded to weeks 1 and 4 after the intervention for the immediate treatment group. Secondary outcomes included the Quick Inventory of Depressive Symptomatology-Self Rated (QIDS-SR).
Results: Of the randomized participants, 24 of 27 (89%) completed the intervention and the week 1 and week 4 postsession assessments. This population had a mean (SD) age of 39.8 (12.2) years, was composed of 16 women (67%), and had a mean (SD) baseline GRID-HAMD score of 22.8 (3.9). The mean (SD) GRID-HAMD scores at weeks 1 and 4 (8.0 [7.1] and 8.5 [5.7]) in the immediate treatment group were statistically significantly lower than the scores at the comparable time points of weeks 5 and 8 (23.8 [5.4] and 23.5 [6.0]) in the delayed treatment group. The effect sizes were large at week 5 (Cohen d = 2.5; 95% CI, 1.4-3.5; P < .001) and week 8 (Cohen d = 2.6; 95% CI, 1.5-3.7; P < .001). The QIDS-SR documented a rapid decrease in mean (SD) depression score from baseline to day 1 after session 1 (16.7 [3.5] vs 6.3 [4.4]; Cohen d = 2.6; 95% CI, 1.8-3.5; P < .001), which remained statistically significantly reduced through the week 4 follow-up (6.0 [5.7]; Cohen d = 2.3; 95% CI, 1.5-3.0; P < .001). In the overall sample, 17 participants (71%) at week 1 and 17 (71%) at week 4 had a clinically significant response to the intervention (≥50% reduction in GRID-HAMD score), and 14 participants (58%) at week 1 and 13 participants (54%) at week 4 were in remission (≤7 GRID-HAMD score).
Conclusions and relevance: Findings suggest that psilocybin with therapy is efficacious in treating MDD, thus extending the results of previous studies of this intervention in patients with cancer and depression and of a nonrandomized study in patients with treatment-resistant depression.
Trial registration: ClinicalTrials.gov Identifier: NCT03181529.
Conflict of interest statement
Figures


Comment in
-
Psilocybin-Assisted Supportive Psychotherapy in the Treatment of Major Depression-Quo Vadis?JAMA Psychiatry. 2021 May 1;78(5):476-478. doi: 10.1001/jamapsychiatry.2020.2901. JAMA Psychiatry. 2021. PMID: 33146675 No abstract available.
-
Errors in a Response Rate and in Effect Sizes in Study of Psilocybin-Assisted Therapy for Major Depressive Disorder.JAMA Psychiatry. 2021 May 1;78(5):569. doi: 10.1001/jamapsychiatry.2020.4638. JAMA Psychiatry. 2021. PMID: 33566073 No abstract available.
References
-
- World Health Organization . Depression fact sheet. World Health Organization. Published December 2019. Accessed January 11, 2020. https://www.who.int/mediacentre/factsheets/fs369/en/
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

