Using a Composite Maternal-Infant Outcome Measure in Tuberculosis-Prevention Studies Among Pregnant Women
- PMID: 33146706
- PMCID: PMC8326545
- DOI: 10.1093/cid/ciaa1674
Using a Composite Maternal-Infant Outcome Measure in Tuberculosis-Prevention Studies Among Pregnant Women
Abstract
Background: Tuberculosis (TB-)-preventive therapy (TPT) among pregnant women reduces risk of TB in mothers and infants, but timing of initiation should consider potential adverse effects. We propose an analytical approach to evaluate the risk-benefit of interventions.
Methods: A novel outcome measure that prioritizes maternal and infant events was developed with a 2-stage Delphi survey, where a panel of stakeholders assigned scores from 0 (best) to 100 (worst) based on perceived desirability. Using data from TB APPRISE, a trial among pregnant women living with human immunodeficiency virus (WLWH) that randomized the timing of initiation of isoniazid, antepartum versus postpartum, was evaluated.
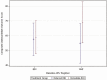
Results: The composite outcome scoring/ranking system categorized mother-infant paired outcomes into 8 groups assigned identical median scores by stakeholders. Maternal/infant TB and nonsevere adverse pregnancy outcomes were assigned similar scores. Mean (SD) composite outcome scores were 43.7 (33.0) and 41.2 (33.7) in the antepartum and postpartum TPT initiation arms, respectively. However, a modifying effect of baseline antiretroviral regimen was detected (P = .049). When women received nevirapine, composite scores were higher (worse outcomes) in the antepartum versus postpartum arms (adjusted difference, 14.3; 95% confidence interval [CI], 2.4-26.2; P = .02), whereas when women received efavirenz there was no difference by timing of TPT (adjusted difference, .62; 95% CI, -3.2-6.2; P = .53).
Conclusions: For TPT, when used by otherwise healthy persons, preventing adverse events is paramount from the perspective of stakeholders. Among pregnant WLWH in high-TB-burden regions, it is important to consider the antepartum antiretroviral regimen taken when deciding when to initiate TPT. Clinical Trials Registration. NCT01494038 (IMPAACT P1078).
Keywords: pregnancy; prioritized composite outcomes; risk-benefit analysis; tuberculosis.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Mafirakureva N. Revisiting the burden of TB in pregnant and post-partum women (abstract SP-53-C6). Abstract book: 50th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease (The Union). Int J Tuberc Lung Dis 2019; 23:S49.
-
- Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012; 185:779–84. - PubMed
-
- World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, Switzerland: World Health Organization, 2018. - PubMed

