Pharmacokinetics and Safety of Single and Multiple Doses of Oral N-Acetylcysteine in Healthy Chinese and Caucasian Volunteers: An Open-Label, Phase I Clinical Study
- PMID: 33146843
- PMCID: PMC7854405
- DOI: 10.1007/s12325-020-01542-4
Pharmacokinetics and Safety of Single and Multiple Doses of Oral N-Acetylcysteine in Healthy Chinese and Caucasian Volunteers: An Open-Label, Phase I Clinical Study
Abstract
Introduction: Few studies have evaluated whether the pharmacokinetics of N-acetyl-cysteine (NAC) are different in Chinese and Caucasian individuals.
Methods: This single- and multiple-dose, single-centre, open-label, phase I clinical study was conducted in healthy adult volunteers. All participants received oral NAC 600-mg uncoated tablets, which were administered first as a single dose and, following a 48-h wash-out period, twice daily for 3 days. Blood and urine were collected after single- and multiple-dose NAC administration. Adverse event (AE) data were collected throughout the study.
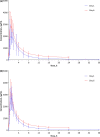
Results: Fifteen Chinese and 15 Caucasian (mostly Italian) individuals (males 66.7%, mean age 36.8 years) participated in the study. Pharmacokinetic characteristics of NAC were similar in the two cohorts. Following both single- and multiple-dose administration, plasma concentration of NAC increased rapidly, reaching a peak at approximately 1.0 h. Maximum plasma concentration and extent of exposure were higher after multiple doses than after a single dose. The accumulation ratio was relatively consistent in both Chinese (mean ± standard deviation 1.5 ± 0.4) and Caucasian (1.4 ± 0.2) participants. The half-life was 15.4 h in Chinese and 18.7 h in Caucasian participants, and the fraction of NAC excreted in urine in the 36 h following administration was 3.7% in Chinese and 3.8% in Caucasian participants. Two Caucasian participants had a total of 3 AEs (headache, presyncope and dysmenorrhoea). No AEs occurred in Chinese participants.
Conclusions: The pharmacokinetic characteristics of NAC are similar in healthy Chinese and Caucasian individuals after single and repeated administration. NAC has a favourable tolerability profile.
Keywords: Caucasian; Chinese; Healthy volunteer; N-acetylcysteine; Pharmacokinetics; Phase I.
Figures
Similar articles
-
Phase I study of the pharmacokinetics and safety of single and multiple doses of intravenous N-acetylcysteine in healthy Chinese subjects.Eur Rev Med Pharmacol Sci. 2023 Dec;27(24):12103-12111. doi: 10.26355/eurrev_202312_34808. Eur Rev Med Pharmacol Sci. 2023. PMID: 38164872 Clinical Trial.
-
Pharmacokinetics, Safety Profile, and Tolerability of Tetramethylpyrazine Nitrone Tablets After Single and Multiple Ascending Doses in Healthy Chinese Volunteers.Eur J Drug Metab Pharmacokinet. 2024 Mar;49(2):207-217. doi: 10.1007/s13318-024-00877-5. Epub 2024 Feb 21. Eur J Drug Metab Pharmacokinet. 2024. PMID: 38381348 Clinical Trial.
-
P2X3 Receptor Antagonist Eliapixant in Phase I Clinical Trials: Safety and Inter-ethnic Comparison of Pharmacokinetics in Healthy Chinese and Japanese Participants.Clin Pharmacokinet. 2024 Jun;63(6):901-915. doi: 10.1007/s40262-024-01387-y. Epub 2024 Jun 21. Clin Pharmacokinet. 2024. PMID: 38907175 Clinical Trial.
-
Tolerability and pharmacokinetics of ranolazine following single and multiple sustained-release doses in Chinese healthy adult volunteers: a randomized, open-label, Latin square design, phase I study.Am J Cardiovasc Drugs. 2013 Feb;13(1):17-25. doi: 10.1007/s40256-013-0006-7. Am J Cardiovasc Drugs. 2013. PMID: 23355361 Clinical Trial.
-
Safety of N-Acetylcysteine at High Doses in Chronic Respiratory Diseases: A Review.Drug Saf. 2021 Mar;44(3):273-290. doi: 10.1007/s40264-020-01026-y. Epub 2020 Dec 16. Drug Saf. 2021. PMID: 33326056 Free PMC article. Review.
Cited by
-
Intestinal permeability of N-acetylcysteine is driven by gut microbiota-dependent cysteine palmitoylation.Nat Commun. 2025 May 19;16(1):4623. doi: 10.1038/s41467-025-59916-7. Nat Commun. 2025. PMID: 40389439 Free PMC article.
-
'Comprehensive review of emerging drug targets in traumatic brain injury (TBI): challenges and future scope.Inflammopharmacology. 2024 Oct;32(5):3271-3293. doi: 10.1007/s10787-024-01524-w. Epub 2024 Jul 17. Inflammopharmacology. 2024. PMID: 39023681 Review.
-
Premature ovarian insufficiency: a review on the role of oxidative stress and the application of antioxidants.Front Endocrinol (Lausanne). 2023 Aug 1;14:1172481. doi: 10.3389/fendo.2023.1172481. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37600717 Free PMC article. Review.
-
N-Acetylcysteine (NAC): Impacts on Human Health.Antioxidants (Basel). 2021 Jun 16;10(6):967. doi: 10.3390/antiox10060967. Antioxidants (Basel). 2021. PMID: 34208683 Free PMC article. Review.
-
N-acetylcysteine in Kidney Disease: Molecular Mechanisms, Pharmacokinetics, and Clinical Effectiveness.Kidney Int Rep. 2024 Jul 20;9(10):2883-2903. doi: 10.1016/j.ekir.2024.07.020. eCollection 2024 Oct. Kidney Int Rep. 2024. PMID: 39430194 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources