Oxygen transport kinetics underpin rapid and robust diaphragm recovery following chronic spinal cord injury
- PMID: 33146892
- PMCID: PMC7894160
- DOI: 10.1113/JP280684
Oxygen transport kinetics underpin rapid and robust diaphragm recovery following chronic spinal cord injury
Abstract
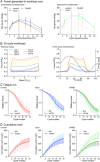
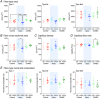
Key points: Spinal treatment can restore diaphragm function in all animals 1 month following C2 hemisection induced paralysis. Greater recovery occurs the longer after injury the treatment is applied. Through advanced assessment of muscle mechanics, innovative histology and oxygen tension modelling, we have comprehensively characterized in vivo diaphragm function and phenotype. Muscle work loops reveal a significant deficit in diaphragm functional properties following chronic injury and paralysis, which are normalized following restored muscle activity caused by plasticity-induced spinal reconnection. Injury causes global and local alterations in diaphragm muscle vascular supply, limiting oxygen diffusion and disturbing function. Restoration of muscle activity reverses these alterations, restoring oxygen supply to the tissue and enabling recovery of muscle functional properties. There remain metabolic deficits following restoration of diaphragm activity, probably explaining only partial functional recovery. We hypothesize that these deficits need to be resolved to restore complete respiratory motor function.
Abstract: Months after spinal cord injury (SCI), respiratory deficits remain the primary cause of morbidity and mortality for patients. It is possible to induce partial respiratory motor functional recovery in chronic SCI following 2 weeks of spinal neuroplasticity. However, the peripheral mechanisms underpinning this recovery are largely unknown, limiting development of new clinical treatments with potential for complete functional restoration. Utilizing a rat hemisection model, diaphragm function and paralysis was assessed and recovered at chronic time points following trauma through chondroitinase ABC induced neuroplasticity. We simulated the diaphragm's in vivo cyclical length change and activity patterns using the work loop technique at the same time as assessing global and local measures of the muscles histology to quantify changes in muscle phenotype, microvascular composition, and oxidative capacity following injury and recovery. These data were fed into a physiologically informed model of tissue oxygen transport. We demonstrate that hemidiaphragm paralysis causes muscle fibre hypertrophy, maintaining global oxygen supply, although it alters isolated muscle kinetics, limiting respiratory function. Treatment induced recovery of respiratory activity normalized these effects, increasing oxygen supply, restoring optimal diaphragm functional properties. However, metabolic demands of the diaphragm were significantly reduced following both injury and recovery, potentially limiting restoration of normal muscle performance. The mechanism of rapid respiratory muscle recovery following spinal trauma occurs through oxygen transport, metabolic demand and functional dynamics of striated muscle. Overall, these data support a systems-wide approach to the treatment of SCI, and identify new targets to mediate complete respiratory recovery.
Keywords: angiogenesis; capillary domain area; diaphragm; mechanical properties; spinal cord injury.
© 2020 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures








Comment in
-
Muscling in on neurorehabilitative strategies to counter respiratory motor dysfunction in cervical spinal cord injury.J Physiol. 2021 Feb;599(4):1009-1010. doi: 10.1113/JP281042. Epub 2020 Dec 4. J Physiol. 2021. PMID: 33226628 No abstract available.
Similar articles
-
Protein Tyrosine Phosphatase σ Inhibitory Peptide Promotes Recovery of Diaphragm Function and Sprouting of Bulbospinal Respiratory Axons after Cervical Spinal Cord Injury.J Neurotrauma. 2020 Feb 1;37(3):572-579. doi: 10.1089/neu.2019.6586. Epub 2019 Sep 18. J Neurotrauma. 2020. PMID: 31392919 Free PMC article.
-
Adenosine A1 receptor mRNA expression and the effects of systemic theophylline administration on respiratory function 4 months after C2 hemisection.J Spinal Cord Med. 2003 Winter;26(4):364-71. doi: 10.1080/10790268.2003.11753707. J Spinal Cord Med. 2003. PMID: 14992338
-
AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury.FASEB J. 2019 Dec;33(12):13775-13793. doi: 10.1096/fj.201901730R. Epub 2019 Oct 2. FASEB J. 2019. PMID: 31577916 Free PMC article.
-
Adenosinergic mechanisms underlying recovery of diaphragm motor function following upper cervical spinal cord injury: potential therapeutic implications.Neurol Res. 2005 Mar;27(2):195-205. doi: 10.1179/016164105X21977. Neurol Res. 2005. PMID: 15829183 Review.
-
The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury.J Appl Physiol (1985). 2003 Feb;94(2):795-810. doi: 10.1152/japplphysiol.00847.2002. J Appl Physiol (1985). 2003. PMID: 12531916 Review.
Cited by
-
The importance of muscle activation on the interpretation of muscle mechanical performance.J Exp Biol. 2024 Nov 1;227(21):jeb248051. doi: 10.1242/jeb.248051. Epub 2024 Nov 8. J Exp Biol. 2024. PMID: 39369302 Free PMC article.
-
Contrasting Experimental Rodent Aftercare With Human Clinical Treatment for Cervical Spinal Cord Injury: Bridging the Translational "Valley of Death".J Neurotrauma. 2023 Dec;40(23-24):2469-2486. doi: 10.1089/neu.2023.0314. Epub 2023 Nov 10. J Neurotrauma. 2023. PMID: 37772694 Free PMC article. Review.
-
Recovery of Forearm and Fine Digit Function After Chronic Spinal Cord Injury by Simultaneous Blockade of Inhibitory Matrix Chondroitin Sulfate Proteoglycan Production and the Receptor PTPσ.J Neurotrauma. 2023 Dec;40(23-24):2500-2521. doi: 10.1089/neu.2023.0117. Epub 2023 Oct 11. J Neurotrauma. 2023. PMID: 37606910 Free PMC article.
-
Accessory respiratory muscles performance among people with spinal cord injury while singing songs with different musical parameters.PLoS One. 2024 Jul 5;19(7):e0305940. doi: 10.1371/journal.pone.0305940. eCollection 2024. PLoS One. 2024. PMID: 38968230 Free PMC article.
-
Heterogeneity in form and function of the rat extensor digitorum longus motor unit.J Anat. 2022 Apr;240(4):700-710. doi: 10.1111/joa.13590. Epub 2021 Nov 10. J Anat. 2022. PMID: 34761377 Free PMC article.
References
-
- Al‐Shammari AA, Kissane RWP, Holbek S, Mackey AL, Andersen TR, Gaffney EA, Kjaer M & Egginton S (2019). Integrated method for quantitative morphometry and oxygen transport modeling in striated muscle. J Appl Physiol 126, 544–557. - PubMed
-
- Alilain WJ & Goshgarian HG (2008). Glutamate receptor plasticity and activity‐regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol 212, 348–357. - PMC - PubMed
-
- Altringham JD & Young IS (1991). Power output and the frequency of oscillatory work in mammalian diaphragm muscle: the effects of animal size. J Exp Biol 157, 381–389. - PubMed
-
- Ameredes BT, Zhan WZ, Prakash YS, Vandenboom R & Sieck GC (2000). Power fatigue of the rat diaphragm muscle. J Appl Physiol 89, 2215–2219. - PubMed
-
- Askew GN & Marsh RL (1997). The effects of length trajectory on the mechanical power output of mouse skeletal muscles. J Exp Biol 200, 3119–3131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

