Autophagic receptor p62 protects against glycation-derived toxicity and enhances viability
- PMID: 33146912
- PMCID: PMC7681057
- DOI: 10.1111/acel.13257
Autophagic receptor p62 protects against glycation-derived toxicity and enhances viability
Abstract
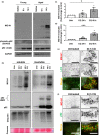
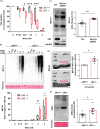
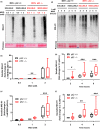
Diabetes and metabolic syndrome are associated with the typical American high glycemia diet and result in accumulation of high levels of advanced glycation end products (AGEs), particularly upon aging. AGEs form when sugars or their metabolites react with proteins. Associated with a myriad of age-related diseases, AGEs accumulate in many tissues and are cytotoxic. To date, efforts to limit glycation pharmacologically have failed in human trials. Thus, it is crucial to identify systems that remove AGEs, but such research is scanty. Here, we determined if and how AGEs might be cleared by autophagy. Our in vivo mouse and C. elegans models, in which we altered proteolysis or glycative burden, as well as experiments in five types of cells, revealed more than six criteria indicating that p62-dependent autophagy is a conserved pathway that plays a critical role in the removal of AGEs. Activation of autophagic removal of AGEs requires p62, and blocking this pathway results in accumulation of AGEs and compromised viability. Deficiency of p62 accelerates accumulation of AGEs in soluble and insoluble fractions. p62 itself is subject to glycative inactivation and accumulates as high mass species. Accumulation of p62 in retinal pigment epithelium is reversed by switching to a lower glycemia diet. Since diminution of glycative damage is associated with reduced risk for age-related diseases, including age-related macular degeneration, cardiovascular disease, diabetes, Alzheimer's, and Parkinson's, discovery of methods to limit AGEs or enhance p62-dependent autophagy offers novel potential therapeutic targets to treat AGEs-related pathologies.
Keywords: aging; autophagy; glycative stress; p62; proteotoxicity.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bejarano, E. , Girao, H. , Yuste, A. , Patel, B. , Marques, C. , Spray, D. C. , Pereira, P. , & Cuervo, A. M. (2012). Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin‐dependent manner. Molecular Biology of the Cell, 23(11), 2156‐2169. 10.1091/mbc.E11-10-0844 - DOI - PMC - PubMed
-
- Bejarano, E. , Murray, J. W. , Wang, X. , Pampliega, O. , Yin, D. , Patel, B. , Yuste, A. , Wolkoff, A. W. , & Cuervo, A. M. (2018). Defective recruitment of motor proteins to autophagic compartments contributes to autophagic failure in aging. Aging Cell, 17(4), e12777 10.1111/acel.12777 - DOI - PMC - PubMed

