Rapid diagnostic tests for Plasmodium vivax malaria in endemic countries
- PMID: 33146932
- PMCID: PMC8078698
- DOI: 10.1002/14651858.CD013218.pub2
Rapid diagnostic tests for Plasmodium vivax malaria in endemic countries
Abstract
Background: Plasmodium vivax (P vivax) is a focus of malaria elimination. It is important because P vivax and Plasmodium falciparum infection are co-endemic in some areas. There are asymptomatic carriers of P vivax, and the treatment for P vivax and Plasmodium ovale malaria differs from that used in other types of malaria. Rapid diagnostic tests (RDTs) will help distinguish P vivax from other malaria species to help treatment and elimination. There are RDTs available that detect P vivax parasitaemia through the detection of P vivax-specific lactate dehydrogenase (LDH) antigens.
Objectives: To assess the diagnostic accuracy of RDTs for detecting P vivax malaria infection in people living in malaria-endemic areas who present to ambulatory healthcare facilities with symptoms suggestive of malaria; and to identify which types and brands of commercial tests best detect P vivax malaria.
Search methods: We undertook a comprehensive search of the following databases up to 30 July 2019: Cochrane Infectious Diseases Group Specialized Register; Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE (PubMed); Embase (OVID); Science Citation Index Expanded (SCI-EXPANDED) and Conference Proceedings Citation Index-Science (CPCI-S), both in the Web of Science.
Selection criteria: Studies comparing RDTs with a reference standard (microscopy or polymerase chain reaction (PCR)) in blood samples from patients attending ambulatory health facilities with symptoms suggestive of malaria in P vivax-endemic areas.
Data collection and analysis: For each included study, two review authors independently extracted data using a pre-piloted data extraction form. The methodological quality of the studies were assessed using a tailored Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. We grouped studies according to commercial brand of the RDT and performed meta-analysis when appropriate. The results given by the index tests were based on the antibody affinity (referred to as the strength of the bond between an antibody and an antigen) and avidity (referred to as the strength of the overall bond between a multivalent antibody and multiple antigens). All analyses were stratified by the type of reference standard. The bivariate model was used to estimate the pooled sensitivity and specificity with 95% confidence intervals (CIs), this model was simplified when studies were few. We assessed the certainty of the evidence using the GRADE approach.
Main results: We included 10 studies that assessed the accuracy of six different RDT brands (CareStart Malaria Pf/Pv Combo test, Falcivax Device Rapid test, Immuno-Rapid Malaria Pf/Pv test, SD Bioline Malaria Ag Pf/Pv test, OnSite Pf/Pv test and Test Malaria Pf/Pv rapid test) for detecting P vivax malaria. One study directly compared the accuracy of two RDT brands. Of the 10 studies, six used microscopy, one used PCR, two used both microscopy and PCR separately and one used microscopy corrected by PCR as the reference standard. Four of the studies were conducted in Ethiopia, two in India, and one each in Bangladesh, Brazil, Colombia and Sudan. The studies often did not report how patients were selected. In the patient selection domain, we judged the risk of bias as unclear for nine studies. We judged all studies to be of unclear applicability concern. In the index test domain, we judged most studies to be at low risk of bias, but we judged nine studies to be of unclear applicability concern. There was poor reporting on lot testing, how the RDTs were stored, and background parasitaemia density (a key variable determining diagnostic accuracy of RDTs). Only half of the included studies were judged to be at low risk of bias in the reference standard domain, Studies often did not report whether the results of the reference standard could classify the target condition or whether investigators knew the results of the RDT when interpreting the results of the reference standard. All 10 studies were judged to be at low risk of bias in the flow and timing domain. Only two brands were evaluated by more than one study. Four studies evaluated the CareStart Malaria Pf/Pv Combo test against microscopy and two studies evaluated the Falcivax Device Rapid test against microscopy. The pooled sensitivity and specificity were 99% (95% CI 94% to 100%; 251 patients, moderate-certainty evidence) and 99% (95% CI 99% to 100%; 2147 patients, moderate-certainty evidence) for CareStart Malaria Pf/Pv Combo test. For a prevalence of 20%, about 206 people will have a positive CareStart Malaria Pf/Pv Combo test result and the remaining 794 people will have a negative result. Of the 206 people with positive results, eight will be incorrect (false positives), and of the 794 people with a negative result, two would be incorrect (false negative). For the Falcivax Device Rapid test, the pooled sensitivity was 77% (95% CI: 53% to 91%, 89 patients, low-certainty evidence) and the pooled specificity was 99% (95% CI: 98% to 100%, 621 patients, moderate-certainty evidence), respectively. For a prevalence of 20%, about 162 people will have a positive Falcivax Device Rapid test result and the remaining 838 people will have a negative result. Of the 162 people with positive results, eight will be incorrect (false positives), and of the 838 people with a negative result, 46 would be incorrect (false negative).
Authors' conclusions: The CareStart Malaria Pf/Pv Combo test was found to be highly sensitive and specific in comparison to microscopy for detecting P vivax in ambulatory healthcare in endemic settings, with moderate-certainty evidence. The number of studies included in this review was limited to 10 studies and we were able to estimate the accuracy of 2 out of 6 RDT brands included, the CareStart Malaria Pf/Pv Combo test and the Falcivax Device Rapid test. Thus, the differences in sensitivity and specificity between all the RDT brands could not be assessed. More high-quality studies in endemic field settings are needed to assess and compare the accuracy of RDTs designed to detect P vivax.
Copyright © 2020 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
RA has no known conflicts of interest. LC has no known conflicts of interest. SJ has no known conflicts of interest. YT has no known conflicts of interest.
Figures
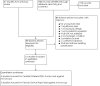




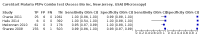

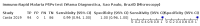
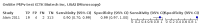

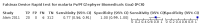
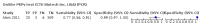


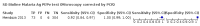
Update of
References
References to studies included in this review
Alam 2011 {published data only}
Chanie 2011 {published data only}
-
- Chanie M, Erko B, Animut A, Legesse M. Performance of CareStart™ Malaria Pf/Pv Combo test for the diagnosis of Plasmodium falciparum and Plasmodium vivax infections in the afar region, North East Ethiopia. Ethiopian Journal of Health Development 2011;25(3):206-11.
Costa 2019 {published data only}
-
- Costa MRF, Barcelos ALR, Camargo MA, Melo GC, Almeida AC, Costa AG, et al. Performance of an immuno-rapid malaria Pf/Pv rapid diagnostic test for malaria diagnosis in the Western Brazilian Amazon. Revista da Sociedade Brasileira de Medicina Tropical 2019;52:e-20170450. - PubMed
Hailu 2014 {published data only}
Mekonnen 2010 {published data only}
-
- Mekonnen Z, Ali S, Belay G, Suleman S, Chatterjee S. Evaluation of the performance of CareStart™ Malaria Pf/Pv Combo rapid diagnostic test for the diagnosis of malaria in Jimma, Southwestern Ethiopia. Acta Tropica 2010;113(3):285-8. - PubMed
Mendoza 2013 {published data only}
-
- Mendoza NM, Cucunubá ZM, Aponte S, González NE, Bernal SD. Field evaluation for diagnostic accuracy of the rapid test SD Bioline Malaria Antigen Pf/Pv® in Colombia [Evaluación de campo de la precisión de la prueba de diagnóstico rápido SD Bioline Malaria Antigen Pf/Pv® en Colombia]. Biomedica 2013;33(4):587-97. - PubMed
Mussa 2019 {published data only}
Saha 2017 {published data only}
-
- Saha S, Narang R, Deshmukh P, Pote K, Anvikar A, Narang P. Diagnostic efficacy of microscopy, rapid diagnostic test and polymerase chain reaction for malaria using bayesian latent class analysis. Indian Journal of Medical Microbiology 2017;35(3):376-80. - PubMed
Sharew 2009 {published data only}
-
- Sharew B, Legesse M, Animut A, Jima D, Medhin G, Erko B. Evaluation of the performance of CareStart™ Malaria Pf/Pv Combo and Paracheck Pf tests for the diagnosis of malaria in Wondo Genet, Southern Ethiopia. Acta Tropica 2009;111(3):321-4. - PubMed
References to studies excluded from this review
Abdelraheem 2016 {published data only}
-
- Abdelraheem MH, Albsheer MM, Mohamed HS, Amin M, Mahdi Abdel Hamid M. Transmission of Plasmodium vivax in Duffy-negative individuals in Central Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2016;110(4):258-60. - PubMed
Adams 2015 {published data only}
-
- Adams M, Joshi SN, Mbambo G, Mu AZ, Roemmich SM, Shrestha B, et al. An ultrasensitive reverse transcription polymerase chain reaction assay to detect asymptomatic low-density Plasmodium falciparum and Plasmodium vivax infections in small volume blood samples. Malaria Journal 2015;14:520. - PMC - PubMed
Adnan 2017 {published data only}
-
- Adnan STA, Warraich RA, Khan MA, Tabassum S. Community based evaluation of malaria rapid diagnostic test (ICT) comparing with conventional method of microscopy. Medical Forum Monthly 2017;28(7):60-63.
Ageep 2013 {published data only}
-
- Ageep AK. Diagnosis of malaria in Red Sea State, Sudan. Annals of Tropical Medicine and Public Health 2013;6(2):232-5.
Andrade 2010 {published data only}
-
- Andrade BB, Reis-Filho A, Barros AM, Souza-Neto SM, Nogueira LL, Fukatano KF, et al. Towards a precise test for malaria diagnosis in the Brazilian Amazon: Comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malaria Journal 2010;9:117. - PMC - PubMed
Arvind 2015 {published data only}
-
- Kumar A. Evaluation of parasite LDH detection strip test (OptiMAL) for rapid diagnosis of malaria in children. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2015;6(1):557-61.
Ashton 2010 {published data only}
Ayorinde 2016 {published data only}
-
- Ayorinde AF, Oyeyiga AM, Nosegbe NO, Folarin OA. A survey of malaria and some arboviral infections among suspected febrile patients visiting a health centre in Simawa, Ogun State, Nigeria. Journal of Infection and Public Health 2016;9(1):52-9. - PubMed
Ba 2017 {published data only}
-
- Ba H, Ahouidi AD, Duffy CW, Deh YB, Diedhiou C, Tandia A, et al. Evaluation of malaria rapid diagnostic test Optimal-IT® pLDH along the Plasmodium falciparum distribution limit in Mauritania [Evaluation du test de diagnostic rapide du paludisme OptiMal-IT® pLDH à la limite de la distribution de Plasmodium falciparum en Mauritanie]. Bulletin de la Societe de pathologie exotique 2017;110(1):31-7. - PubMed
Bahk 2018 {published data only}
Barber 2013 {published data only}
-
- Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. Journal of Clinical Microbiology 2013;51(4):1118–23. - PMC - PubMed
Bell 2001 {published data only}
Bendezu 2010 {published data only}
Berhane 2017 {published data only}
Berzosa 2018 {published data only}
Bharti 2008 {published data only}
Bharti 2013 {published data only}
-
- Bharti PK, Chand SK, Singh MP, Mishra S, Shukla MM, Singh R, et al. Emergence of a new focus of Plasmodium malariae in forest villages of District Balaghat, Central India: Implications for the diagnosis of malaria and its control. Tropical Medicine and International Health 2013;18(1):12-7. - PubMed
Bhide 2014 {published data only}
-
- Bhide M, Parekh V. Automated screening for malaria parasites on Mindray BC-6800 Hematology Analyzer: A follow-up report. International Journal of Laboratory Hematology 2014;36:80-1.
Birhanie 2016 {published data only}
Bisoffi 2014 {published data only}
-
- Bisoffi Z, Gobbi F, Van den Ende J. Rapid diagnostic tests for malaria. BMJ 2014;348:g3846. - PubMed
Britton 2016 {published data only}
-
- Britton S, Cheng Q, Grigg MJ, Poole CB, Pasay C, William T, et al. Sensitive Detection of Plasmodium vivax Using a High-Throughput, Colourimetric Loop Mediated Isothermal Amplification (HtLAMP) Platform: A Potential Novel Tool for Malaria Elimination. PLoS Neglected Tropical Diseases 2016;10(2):e0004443. - PMC - PubMed
Chayani 2004 {published data only}
-
- Chayani N, Das B, Sur M, Bajoria S. Comparison of parasite lactate dehydrogenase based immunochromatographic antigen detection assay (optimal) with microscopy for detection of malaria parasites. Indian Journal of Medical Microbiology 2004;22(2):104-6. - PubMed
Cho 2016 {published data only}
Dahesh 2015 {published data only}
-
- Dahesh SM, Mostafa HI. Reevaluation of malaria parasites in El-Fayoum Governorate, Egypt using Rapid Diagnostic Tests (RDTs). Journal of the Egyptian Society of Parasitology 2015;45(3):617-28. - PubMed
Dash 2013 {published data only}
-
- Dash M. Comparison of rapid immunochromatographic assays (ICT malaria P.f./P.v. test and OptiMAL test) with microscopy for detection of malaria parasites. International Journal of Pharma and Bio Sciences 2013;4(4):322-7.
Deida 2019 {published data only}
DeKoninck 2017 {published data only}
Dev 2004 {published data only}
-
- Dev V. Relative utility of dipsticks for diagnosis of malaria in mesoendemic area for Plasmodium falciparum and P. vivax in Northeastern India. Vector Borne and Zoonotic Diseases 2004;4(2):123-30. - PubMed
Dinzouna‐Boutamba 2014 {published data only}
Dzakah 2014 {published data only}
Ehtesham 2015 {published data only}
Eibach 2013 {published data only}
Elahi 2013 {published data only}
Endeshaw 2012 {published data only}
Falade 2016 {published data only}
-
- Falade CO, Ajayi IO, Nsungwa-Sabiiti J, Siribie M, Diarra A, Serme L, et al. Malaria Rapid Diagnostic Tests and Malaria Microscopy for Guiding Malaria Treatment of Uncomplicated Fevers in Nigeria and Prereferral Cases in 3 African Countries. Clinical Infectious Diseases 2016;63:S290-s297. - PMC - PubMed
Fernando 2004 {published data only}
-
- Fernando SD, Karunaweera ND, Fernando WP, Attanayake N, Wickremasinghe AR. A cost analysis of the use of the rapid, whole-blood, immunochromatographic P.f/P.v assay for the diagnosis of Plasmodium vivax malaria in rural areas of Sri Lanka. Annals of Tropical Medicine and Parasitology 2004;98(1):5-13. - PubMed
Fernando 2004a {published data only}
-
- Fernando SD, Karunaweera ND, Fernando WP. Evaluation of a rapid whole blood immunochromatographic assay for the diagnosis of Plasmodium falciparum and Plasmodium vivax malaria. Ceylon Medical Journal 2004;49(1):7-11. - PubMed
Foster 2014 {published data only}
Fransisca 2015 {published data only}
-
- Fransisca L, Kusnanto JH, Satoto TB, Sebayang B, Supriyanto, Andriyan E, et al. Comparison of rapid diagnostic test Plasmotec Malaria-3, microscopy, and quantitative real-time PCR for diagnoses of Plasmodium falciparum and Plasmodium vivax infections in Mimika Regency, Papua, Indonesia. Malaria Journal 2015;14:103. - PMC - PubMed
Gabrielli 2016 {published data only}
Ghai 2016 {published data only}
Gupta 2018 {published data only}
-
- Gupta P Gupta P, Rao S, Singh N, Kalita D. Comparison between microscopy and rapid diagnostic tests in diagnosis of malaria at a tertiary care medical institution in Uttarakhand (A 3-year study). Asian Journal of Pharmaceutical and Clinical Research 2018;11(2):94-6.
Harani 2006 {published data only}
-
- Harani MS, Beg MA, Khaleeq L, Adil SN, Kakepoto GN, Khurshid M. Role of ICT malaria immunochromatographic test for rapid diagnosis of malaria. Journal of the Pakistan Medical Association 2006;56(4):167-71. - PubMed
Hawash 2019 {published data only}
Jabeen 2016 {published data only}
-
- Jabeen S, Farrukh U, Hameed SA, Kanwal S, Qayyum M. An investigation on the prevalence and efficiency of immunochromatographic testing in suspected malarial patients of Rawalpindi and Islamabad, Pakistan. Turkish Journal of Medical Sciences 2016;46(5):1329-34. - PubMed
Jahan 2019 {published data only}
Joseph 2018 {published data only}
-
- Joseph N, Uchila AK. Validation of malaria antigen detecting rapid diagnostic test kit: A study from highly endemic area in Coastal India. Journal of Clinical and Diagnostic Research 2018;12(9):16-20.
Karimov 2013 {published data only}
-
- Karimov SS, Saiburkhonov DS. Evaluation of the efficiency of rapid tests in identifying malaria patients and parasite carriers in the Republic of Tajikistan. Meditsinskaia parazitologiia i parazitarnye bolezni 2013;(1):44-5. - PubMed
Kim 2013 {published data only}
Kolaczinski 2004 {published data only}
-
- Klolaczinski J, Mohammed N, Ali I, Ali M, Khan N, Ezard N, et al. Comparison of the OptiMAL rapid antigen test with field microscopy for the detection of Plasmodium vivax and P. falciparum: Considerations for the application of the rapid test in Afghanistan. Annals of Tropical Medicine and Parasitology 2004;98(1):15-20. - PubMed
Kosack 2013 {published data only}
Kumari 2014 {published data only}
-
- Kumari PR. A study of evaluation of rapid diagnostic techniques of Malaria in Urban Slums of Vijayawada, Krishna District, Andhra Pradesh, India. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(4):1428-49.
Liu 2013 {published data only}
-
- Liu H, Li XR, Li CF, Li XL, Wang HY, Nie RH. Field evaluation of SD(BIOLINE) malaria antigen Plasmodium falciparum/Plasmodium vivax rapid test kit. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2013;31(2):160-1. - PubMed
Mallepaddi 2019 {published data only}
Metzger 2011 {published data only}
-
- Metzger WG, Vivas-Martínez S, Giron A, Vaccari E, Campos E, Rodríguez I, et al. Assessment of routine malaria diagnosis in the Venezuelan Amazon. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011;105(5):262-8. - PubMed
Moges 2012 {published data only}
Mohon 2012 {published data only}
Olasehinde 2018 {published data only}
Pakalapati 2013 {published data only}
-
- Pakalapati D, Garg S, Middha S, Kochar A, Subudhi AK, Arunachalam BP, et al. Comparative evaluation of microscopy, OptiMAL(®) and 18S rRNA gene based multiplex PCR for detection of Plasmodium falciparum & Plasmodium vivax from field isolates of Bikaner, India. Asian Pacific Journal of Tropical Medicine 2013;6(5):346-51. - PubMed
Pattanasin 2003 {published data only}
-
- Pattanasin S, Proux S, Chompasuk D, Luwiradaj K, Jacquier P, Looareesuwan S, et al. Evaluation of a new Plasmodium lactate dehydrogenase assay (OptiMAL-IT) for the detection of malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(6):672-4. - PubMed
Puri 2013 {published data only}
-
- Puri B, Mehta P, Ingole NA, Prasad P, Mathure T. Laboratory tests for malaria: A diagnostic conundrum? South African Medical Journal 2013;103(9):625-7. - PubMed
Rakotonirina 2008 {published data only}
-
- Rakotonirina H, Barnadas C, Raherijafy R, Andrianantenaina H, Ratsimbasoa A, Randrianasolo L, et al. Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria-endemic countries. American Journal of Tropical Medicine and Hygiene 2008;78(2):217-21. - PubMed
Ranjan 2016 {published data only}
-
- Ranjan P, Ghoshal U. Utility of nested polymerase chain reaction over the microscopy and immuno-chromatographic test in the detection of Plasmodium species and their clinical spectrum. Parasitology Research 2016;115(9):3375-85. - PubMed
Ratsimbasoa 2007 {published data only}
-
- Ratsimbasoa A, Randriamanantena A, Raherinjafy R, Rasoarilalao N, Ménard D. Which malaria rapid test for Madagascar? Field and laboratory evaluation of three test and expert microscopy of samples from suspected malaria patients in Madagascar. American Journal of Tropical Medicine and Hygiene 2007;76(3):481-5. - PubMed
Ratsimbasoa 2008 {published data only}
-
- Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Ménard D. Evaluation of two new immunochromatographic assays for diagnosis of malaria. American Journal of Tropical Medicine and Hygiene 2008;79(5):670-2. - PubMed
Samane 2010 {published data only}
-
- Samane AK, Nahid HZ, Saaed S, Khazen H, Ali H, Ahmad R, et al. Comparison of microscopy and RDTs techniques for laboratory detection of malaria. African Journal of Biotechnology 2010;9(10):1514-6.
Selimuzzaman 2010 {published data only}
-
- Selimuzzaman SM, Islam SJ, Nahar Z, Das R, Rahman MA, Rahman MA. Malariagen malaria Pf/Pv antigen rapid test: A simple and effective tool for diagnosis of malaria in the far-flung hilly areas of Bangladesh. Mymensingh Medical Journal 2010;19(1):94-9. - PubMed
Shaikh 2013 {published data only}
Sharma 2014 {published data only}
-
- Sharma J. Prevalence of malaria cases in tea garden areas of Lakhimpur District, Assam. International Journal of Pharmacy and Pharmaceutical Sciences 2014;6(8):571-3.
Singh 2000a {published data only}
-
- Singh N, Saxena A, Valecha N. Field evaluation of the ICT malaria P.f/P.v immunochromatographic test for diagnosis of Plasmodium falciparum and P. vivax infection in forest villages in Chhindwara, Central India. Tropical Medicine and International Health 2000;5(11):765-70. - PubMed
Singh 2003 {published data only}
-
- Singh N, Valecha N, Nagpal AC, Mishra SS, Varma HS, Subbaro SK. The hospital- and field-based performances of the OptiMAL test, for malaria diagnosis and treatment monitoring in Central India. Annals of Tropical Medicine and Parasitology 2003;97(1):5-13. - PubMed
Singh 2013 {published data only}
Siwal 2018 {published data only}
Stijnberg 2013 {published data only}
-
- Stijnberg D, Cairo H. Rapid diagnostic test (RDT) performance of the malaria gold mining program in Suriname: Comparing the performance of two RDT's. In: 62nd Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2013 13-17 November; Washington, DC. Vol. 89(5 Suppl 1). Washington, DC, USA: The American Society of Tropical Medicine and Hygiene, 2013:384.
Strom 2014 {published data only}
Thongdee 2014 {published data only}
Tjitra 1999 {published data only}
-
- Tjitra E, Suprianto S, Dyer M, Currie BJ, Anstey NM. Field evaluation of the ICT malaria P.f/P.v immunochromatographic test in detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in Eastern Indonesia. Journal of Clinical Microbiology 1999;37(8):2412-7. - PMC - PubMed
Trouvay 2013 {published data only}
Valecha 2003 {published data only}
-
- Valecha N, Singh N, Yadav RS, Dev V, Aggarwal A, Subbarao SK. Field evaluation of OptiMAL48 rapid malaria diagnostic test in India. Acta Parasitologica 2003;48(3):229-32.
van den Broek 2006 {published data only}
-
- den Broek I, Hill O, Gordillo F, Angarita B, Hamade P, Counihan H, et al. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. American Journal of Tropical Medicine and Hygiene 2006;75(6):1209-15. - PubMed
Vohra 2014 {published data only}
-
- Vohra P, Mengi S, Bunger R, Pathania D, Singh VA. Comparison of peripheral blood film stained by Giemsa stain, acridine orange staining and rapid diagnostic tests for detection of P. vivax and P. falciparum in clinically suspected cases of malaria. International Journal of Pharma Medicine and Biological Sciences 2014;3(3):108-112.
Wang 2014 {published data only}
-
- Wang ZY, Jiang L, Zhang YG, Zhang XP, Cai L. Comparison of two rapid diagnostic tests in detection of malaria parasites. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2014;32(1):50-3. - PubMed
Wongsrichanalai 2003 {published data only}
-
- Wongsrichanalai G, Arevalo I, Laoboonchai A, Yingyuen K, Miller RS, Magill AJ, et al. Rapid diagnostic devices for malaria: Field evaluation of a new prototype immunochromatographic assay for the detection of Plasmodium falciparum and non-falciparum Plasmodium. American Journal of Tropical Medicine and Hygiene 2003;69(1):26-30. - PubMed
Woyessa 2013 {published data only}
Xiaodong 2013 {published data only}
References to studies awaiting assessment
Boni 2015 {published data only}
-
- Boni MF, Lover AA, Ngo TD, Nguyen TX, Sutamihardja A, Tran DT, et al. Potential impact of rapid diagnostic test diagnostic error on increased malaria transmission of emerging untreatable malaria. In: 64th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2015 October 25-29; Philadelphia (PA). Vol. 93(4 Suppl). Philadelphia (PA), USA: The American Society of Tropical Medicine and Hygiene, 2015:371.
Cheng 2013 {published data only}
Reda 2016 {published data only}
-
- Reda AG. Evaluation of the performance of SD bioline malaria AG PF/PV and carestartTM malaria PF/PV combo tests for the diagnosis of malaria in two malarious areas in central Ethiopia. In: 65th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2016 November 13-17; Atlanta (GA). Vol. 95(5 Suppl). Atlanta (GA), USA: The American Society of Tropical Medicine and Hygiene, 2016:82-83.
Additional references
Abba 2014
-
- Abba K, Kirkham AJ, Olliaro PL, Deeks JJ, Donegan S, Garner P, et al. Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD011431. [DOI: 10.1002/14651858.CD011431] - DOI - PMC - PubMed
Campo 2015
Chen 2016
Chu 2006
-
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331-2; author reply 1332-3. [PMID: ] - PubMed
Culleton 2012
-
- Culleton R, Carter R. African Plasmodium vivax: Distribution and origins. International Journal for Parasitology 2012;42(12):1091-7. - PubMed
Deeks 2005
-
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882-93. - PubMed
Dobson 1994
-
- Dobson MJ. Malaria in England: A geographical and historical perspective. Parassitologia 1994;36(1-2):35-60. - PubMed
Gatton 2018
GRADE 2013
-
- Schünemann H, Brożek J, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Available from www.gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed 5 December 2017).
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 5 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Han 2017
-
- Han TZ, Han KT, Aye KH, Hlaing T, Thant KZ, Vythilingam I. Comparison of microscopy and PCR for the detection of human Plasmodium species and Plasmodium knowlesi in Southern Myanmar. Asian Pacific Journal of Tropical Biomedicine 2017;7(8):680-5.
Hänscheid 2002
-
- Hänscheid T, Grobusch MP. How useful is PCR in the diagnosis of malaria? Trends in Parasitology 2002;18(9):395-8. - PubMed
Kakkilaya 2003
-
- Kakkilaya BS. Rapid diagnosis of malaria. Laboratory Medicine 2003;34(8):602-8.
Macaskill 2010
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration 2010. Available from: methods.cochrane.org/sdt/handbook-dta-reviews.
Maltha 2013
-
- Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in endemic settings. Clinical Microbiology and Infection 2013;19(5):399-407. - PubMed
Moonasar 2007
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 2016
-
- Roth J, Korevaar D, Leeflang MM, Mens P. Molecular malaria diagnostics: A systematic review and meta-analysis. Critical Reviews in Clinical Laboratory Sciences 2016;53(2):87-105. - PubMed
Shokoples 2009
Snounou 1993
-
- Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Molecular and Biochemical Parasitology 1993;61(2):315-20. - PubMed
STATA 2015 [Computer program]
-
- Stata statistical software. Version 15. College Station, TX: StataCorp LLC, 2017.
Takwoingi 2013
-
- Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544-54. [MEDLINE: ] - PubMed
Takwoingi 2015a
Takwoingi 2015b
Talman 2007
Whiting 2009
-
- Whiting P, Westwood M, Burke M, Sterne J, Glanville J. Systematic reviews of test accuracy should search a range of databases to identify primary studies. Journal of Clinical Epidemiology 2009;61(4):357–64. - PubMed
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. - PubMed
WHO 2003
-
- World Health Organization. Malaria rapid diagnosis:making it work: World Health Organization. Meeting report 20-23 January, Manila. apps.who.int/iris/bitstream/handle/10665/208030/RS_2003_GE_05_PHL_eng.pd... (accessed 30 Oct 2018).
WHO 2006
-
- World Health Organization. Towards quality testing of malaria rapid diagnostic tests: evidence and methods. Proceedings of the WHO Informal Consultation on development and methods for testing malaria rapid diagnostic tests 28 February - 2 March 2006. Available at www.portal.pmnch.org/malaria/publications/atoz/929061238X/en/ (accessed 30 Oct 2018).
WHO 2012
-
- World Health Organization. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: Round 4 (2012). December 2012. Available at www.who.int/malaria/publications/rapid_diagnostic/en/ (accessed 30 Oct 2018).
WHO 2014
-
- World Health Organization, Medicines for Malaria Venture, Roll Back Malaria, and the Wellcome Trust. Severe malaria. Tropical Medicine & International Health 2014;19(s1):7-131.
WHO 2015a
-
- World Health Organization. Guidelines for the treatment of malaria. Third edition. April 2015. http://apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng... (accessed 26 July 2018).
WHO 2015b
-
- World Health Organization. The role of RDTs in malaria control. www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/role_in_malar... (accessed 4 December 2017).
WHO 2017a
-
- World Health Organization. World Malaria Report 2017. apps.who.int/iris/bitstream/handle/10665/259492/9789241565523-eng.pdf;js... (accessed 9 April 2018).
WHO 2017b
-
- World Health Organization. WHO-FIND malaria RDT evaluation programme. Last update 10 July 2017. www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/rdt-evaluatio... (accessed 4 December 2017).
WHO 2017c
-
- World Health Organization. A framework for malaria elimination. Last update March 2017. www.who.int/malaria/publications/atoz/9789241511988/en/ (accessed 29 April 2020).
WHO 2020
-
- World Health Organization. WHO information notice for users. Last updated January 2020. www.who.int/diagnostics_laboratory/procurement/accessbioinc_20012020_not... (accessed 30 July 2020).
Wongsrichanalai 2007
-
- Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT). American Journal of Tropical Medicine and Hygiene 2007;77(6 Suppl):119-27. [PMID: ] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

