Influence of Ionic Strength on Hydrophobic Interactions in Water: Dependence on Solute Size and Shape
- PMID: 33147018
- PMCID: PMC7681779
- DOI: 10.1021/acs.jpcb.0c06399
Influence of Ionic Strength on Hydrophobic Interactions in Water: Dependence on Solute Size and Shape
Abstract
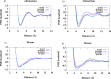
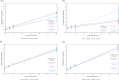
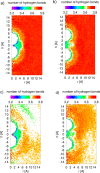
Hydrophobicity is a phenomenon of great importance in biology, chemistry, and biochemistry. It is defined as the interaction between nonpolar molecules or groups in water and their low solubility. Hydrophobic interactions affect many processes in water, for example, complexation, surfactant aggregation, and coagulation. These interactions play a pivotal role in the formation and stability of proteins or biological membranes. In the present study, we assessed the effect of ionic strength, solute size, and shape on hydrophobic interactions between pairs of nonpolar particles. Pairs of methane, neopentane, adamantane, fullerene, ethane, propane, butane, hexane, octane, and decane were simulated by molecular dynamics in AMBER 16.0 force field. As a solvent, TIP3P and TIP4PEW water models were used. Potential of mean force (PMF) plots of these dimers were determined at four values of ionic strength, 0, 0.04, 0.08, and 0.40 mol/dm3, to observe its impact on hydrophobic interactions. The characteristic shape of PMFs with three extrema (contact minimum, solvent-separated minimum, and desolvation maximum) was observed for most of the compounds for hydrophobic interactions. Ionic strength affected hydrophobic interactions. We observed a tendency to deepen contact minima with an increase in ionic strength value in the case of spherical and spheroidal molecules. Additionally, two-dimensional distribution functions describing water density and average number of hydrogen bonds between water molecules were calculated in both water models for adamantane and hexane. It was observed that the density of water did not significantly change with the increase in ionic strength, but the average number of hydrogen bonds changed. The latter tendency strongly depends on the water model used for simulations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Potential of mean force of association of large hydrophobic particles: toward the nanoscale limit.J Phys Chem B. 2010 Jan 21;114(2):993-1003. doi: 10.1021/jp907794h. J Phys Chem B. 2010. PMID: 20039620 Free PMC article.
-
Potential of mean force of hydrophobic association: dependence on solute size.J Phys Chem B. 2007 Sep 13;111(36):10765-74. doi: 10.1021/jp070594t. Epub 2007 Aug 22. J Phys Chem B. 2007. PMID: 17713937
-
Influence of Temperature and Salt Concentration on the Hydrophobic Interactions of Adamantane and Hexane.J Phys Chem B. 2022 Jan 27;126(3):634-642. doi: 10.1021/acs.jpcb.1c09860. Epub 2022 Jan 13. J Phys Chem B. 2022. PMID: 35025490 Free PMC article.
-
Recent developments in the theoretical, simulational, and experimental studies of the role of water hydrogen bonding in hydrophobic phenomena.Adv Colloid Interface Sci. 2016 Sep;235:23-45. doi: 10.1016/j.cis.2016.05.006. Epub 2016 May 18. Adv Colloid Interface Sci. 2016. PMID: 27312562 Review.
-
The Hydrophobic Effects: Our Current Understanding.Molecules. 2022 Oct 18;27(20):7009. doi: 10.3390/molecules27207009. Molecules. 2022. PMID: 36296602 Free PMC article. Review.
Cited by
-
Structural insights into anion selectivity and activation mechanism of LRRC8 volume-regulated anion channels.Cell Rep. 2023 Aug 29;42(8):112926. doi: 10.1016/j.celrep.2023.112926. Epub 2023 Aug 6. Cell Rep. 2023. PMID: 37543949 Free PMC article.
-
Self-Assembling Hydrogel Structures for Neural Tissue Repair.ACS Biomater Sci Eng. 2021 Sep 13;7(9):4136-4163. doi: 10.1021/acsbiomaterials.1c00030. Epub 2021 Mar 29. ACS Biomater Sci Eng. 2021. PMID: 33780230 Free PMC article. Review.
-
Structure determinants defining the specificity of papain-like cysteine proteases.Comput Struct Biotechnol J. 2022 Nov 24;20:6552-6569. doi: 10.1016/j.csbj.2022.11.040. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36467578 Free PMC article. Review.
-
Sticky Science: Using Complex Coacervate Adhesives for Biomedical Applications.Adv Healthc Mater. 2025 Jan;14(2):e2402340. doi: 10.1002/adhm.202402340. Epub 2024 Oct 1. Adv Healthc Mater. 2025. PMID: 39352099 Free PMC article. Review.
-
Synergistic Role of Temperature and Salinity in Aggregation of Nonionic Surfactant-Coated Silica Nanoparticles.Langmuir. 2023 Apr 25;39(16):5917-5928. doi: 10.1021/acs.langmuir.3c00432. Epub 2023 Apr 13. Langmuir. 2023. PMID: 37053432 Free PMC article.
References
-
- Blokzijl W.; Engberts J. B. F. N. Hydrophobic Effects. Opinions and Facts. Angew. Chem., Int. Ed. 1993, 32, 1545–1579. 10.1002/anie.199315451. - DOI
-
- Bartosik A.; Wiśniewska M.; Makowski M. Potentials of Mean Force for Hydrophobic Interactions between Hydrocarbons in Water Solution: Dependence on Temperature, Solute Shape, and Solute Size. J. Phys. Org. Chem. 2015, 28, 10–16. 10.1002/poc.3387. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

