HIV drug resistance profile in South Africa: Findings and implications from the 2017 national HIV household survey
- PMID: 33147285
- PMCID: PMC7641411
- DOI: 10.1371/journal.pone.0241071
HIV drug resistance profile in South Africa: Findings and implications from the 2017 national HIV household survey
Abstract
Background: HIV drug resistance (HIVDR) testing was included in the 2017 South African national HIV household survey. We describe the prevalence of HIVDR by drug class, age, sex and antiretroviral drugs (ARV) status.
Methods: Dried blood were spots tested for HIV, with Viral load (VL), exposure to ARVs and HIVDR testing among those HIV positive. HIVDR testing was conducted on samples with VL ≥1000 copies/ml using Next Generation Sequencing. Weighted percentages of HIVDR are reported.
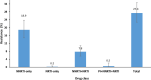
Results: 697/1,105 (63%) of HIV positive samples were sequenced. HIVDR was detected in samples from 200 respondents (27.4% (95% confidence interval (CI) 22.8-32.6)). Among these 130 (18.9% (95% CI 14.8-23.8)), had resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) only, 63 (7.8% (95% CI 5.6-10.9)) resistance to NNRTIs and nucleoside reverse transcriptase inhibitors, and 3 (0.5% (95% CI 0.1-2.1)) resistance to protease inhibitors. Sixty-five (55.7% (95% CI 42.6-67.9) of ARV-positive samples had HIVDR compared to 112 (22.8% (95% CI 17.7-28.7)), in ARV-negative samples. HIVDR was found in 75.6% (95% CI 59.2-87.3), n = 27, samples from respondents who reported ARV use but tested ARV-negative, and in 15.3% (95% CI 6.3-32.8), n = 7, respondents who reported no ARV use and tested ARV-negative. There were no significant age and sex differences in HIVDR.
Conclusion: 27% of virally unsuppressed respondents had HIVDR, increasing to 75% among those who had discontinued ARV. Our findings support strengthening first-line ARV regimens by including drugs with a higher resistance barrier and treatment adherence strategies, and close monitoring of HIVDR.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures
References
-
- UNAIDS, 2019 Global HIV statistics. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_...
-
- Simbayi LC, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, et al. (2018) South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017. Cape Town: HSRC Press
-
- SANAC (South African National Aids Council) (2017). Let Our Actions Count. South Africa’s National Plan on HIV, TB & AIDS and STIs 2017–2022 Pretoria: Department of Health. http://sanac.org.za/wp-content/uploads/2017/05/NSP_FullDocument_FINAL.pdf
-
- UNAIDS (Joint United Nations Programme on HIV/AIDS) (2014a) 90–90–90 An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS
-
- UNAIDS—Global HIV & AIDS statistics—2018 fact sheet. https://www.unaids.org/en/resources/fact-sheet
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

