Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications
- PMID: 33147405
- PMCID: PMC7705895
- DOI: 10.1021/acs.chemrev.0c00451
Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications
Abstract
This is the first comprehensive review on methods and materials for use in optical sensing of pH values and on applications of such sensors. The Review starts with an introduction that contains subsections on the definition of the pH value, a brief look back on optical methods for sensing of pH, on the effects of ionic strength on pH values and pKa values, on the selectivity, sensitivity, precision, dynamic ranges, and temperature dependence of such sensors. Commonly used optical sensing schemes are covered in a next main chapter, with subsections on methods based on absorptiometry, reflectometry, luminescence, refractive index, surface plasmon resonance, photonic crystals, turbidity, mechanical displacement, interferometry, and solvatochromism. This is followed by sections on absorptiometric and luminescent molecular probes for use pH in sensors. Further large sections cover polymeric hosts and supports, and methods for immobilization of indicator dyes. Further and more specific sections summarize the state of the art in materials with dual functionality (indicator and host), nanomaterials, sensors based on upconversion and 2-photon absorption, multiparameter sensors, imaging, and sensors for extreme pH values. A chapter on the many sensing formats has subsections on planar, fiber optic, evanescent wave, refractive index, surface plasmon resonance and holography based sensor designs, and on distributed sensing. Another section summarizes selected applications in areas, such as medicine, biology, oceanography, bioprocess monitoring, corrosion studies, on the use of pH sensors as transducers in biosensors and chemical sensors, and their integration into flow-injection analyzers, microfluidic devices, and lab-on-a-chip systems. An extra section is devoted to current challenges, with subsections on challenges of general nature and those of specific nature. A concluding section gives an outlook on potential future trends and perspectives.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


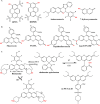
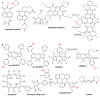
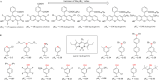
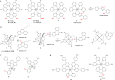











References
-
- Prichard F. E.; Lawn R.; Royal Society of Chemistry (Great Britain) . Measurement of pH: A Practical Handbook; Royal Society of Chemistry: London, 2003.
-
- Wolfbeis O. S.Fiber Optic Chemical Sensors and Biosensors, Vol. 1 and 2; Wolfbeis O. S., Ed.; CRC Press: Boca Raton, FL, 1991.
-
- Lian Y.; Zhang W.; Ding L.; Zhang X.; Zhang Y.; Wang X.. Chapter 8: Nanomaterials for Intracellular pH Sensing and Imaging. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Wang X., Chen X., Eds.; Elsevier, 2019; pp 241–273.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

