Two Aldehyde Clearance Systems Are Essential to Prevent Lethal Formaldehyde Accumulation in Mice and Humans
- PMID: 33147438
- PMCID: PMC7758861
- DOI: 10.1016/j.molcel.2020.10.012
Two Aldehyde Clearance Systems Are Essential to Prevent Lethal Formaldehyde Accumulation in Mice and Humans
Abstract
Reactive aldehydes arise as by-products of metabolism and are normally cleared by multiple families of enzymes. We find that mice lacking two aldehyde detoxifying enzymes, mitochondrial ALDH2 and cytoplasmic ADH5, have greatly shortened lifespans and develop leukemia. Hematopoiesis is disrupted profoundly, with a reduction of hematopoietic stem cells and common lymphoid progenitors causing a severely depleted acquired immune system. We show that formaldehyde is a common substrate of ALDH2 and ADH5 and establish methods to quantify elevated blood formaldehyde and formaldehyde-DNA adducts in tissues. Bone-marrow-derived progenitors actively engage DNA repair but also imprint a formaldehyde-driven mutation signature similar to aging-associated human cancer mutation signatures. Furthermore, we identify analogous genetic defects in children causing a previously uncharacterized inherited bone marrow failure and pre-leukemic syndrome. Endogenous formaldehyde clearance alone is therefore critical for hematopoiesis and in limiting mutagenesis in somatic tissues.
Keywords: DNA damage; ageing; bone marrow failure; cancer; formaldehyde; hematopoiesis; hematopoietic stem cells; immunodeficiency; mutagenesis; oncometabolite.
Copyright © 2020 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

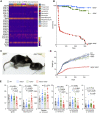



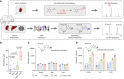
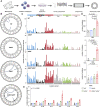

Comment in
-
Formaldehyde Causes Bone Marrow Failure Linked to Transcriptional Reprogramming or Metabolic Deficiency.Mol Cell. 2020 Dec 17;80(6):935-937. doi: 10.1016/j.molcel.2020.11.042. Mol Cell. 2020. PMID: 33338408
References
-
- Bluteau O., Sebert M., Leblanc T., Peffault de Latour R., Quentin S., Lainey E., Hernandez L., Dalle J.-H., Sicre de Fontbrune F., Lengline E. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood. 2018;131:717–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES010126/ES/NIEHS NIH HHS/United States
- G0902418/MRC_/Medical Research Council/United Kingdom
- MC_PC_15065/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_PC_12009/MRC_/Medical Research Council/United Kingdom
- MC_PC_17230/MRC_/Medical Research Council/United Kingdom
- MC_U105178811/MRC_/Medical Research Council/United Kingdom
- MR/M008975/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00016/17/MRC_/Medical Research Council/United Kingdom
- C60150/A23919/CRUK_/Cancer Research UK/United Kingdom
- P42 ES005948/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

