Plasma tau predicts cerebral vulnerability in aging
- PMID: 33147571
- PMCID: PMC7695405
- DOI: 10.18632/aging.104057
Plasma tau predicts cerebral vulnerability in aging
Abstract
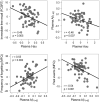
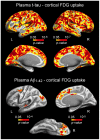
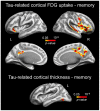
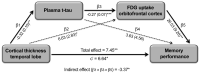
Identifying cerebral vulnerability in late life may help prevent or slow the progression of aging-related chronic diseases. However, non-invasive biomarkers aimed at detecting subclinical cerebral changes in the elderly are lacking. Here, we have examined the potential of plasma total tau (t-tau) for identifying cerebral and cognitive deficits in normal elderly subjects. Patterns of cortical thickness and cortical glucose metabolism were used as outcomes of cerebral vulnerability. We found that increased plasma t-tau levels were associated with widespread reductions of cortical glucose uptake, thinning of the temporal lobe, and memory deficits. Importantly, tau-related reductions of glucose consumption in the orbitofrontal cortex emerged as a determining factor of the relationship between cortical thinning and memory loss. Together, these results support the view that plasma t-tau may serve to identify subclinical cerebral and cognitive deficits in normal aging, allowing detection of individuals at risk for developing aging-related neurodegenerative conditions.
Keywords: FDG-PET; aging; cerebral vulnerability; cortical thickness; plasma tau.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

