Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin
- PMID: 33147690
- PMCID: PMC7663298
- DOI: 10.3390/ijms21218194
Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin
Abstract
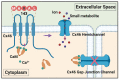
Connexins are the structural components of gap junctions and hemichannels that mediate the communication and exchange of small molecules between cells, and between the intracellular and extracellular environment, respectively. Connexin (Cx) 46 is predominately expressed in lens fiber cells, where they function in maintaining the homeostasis and transparency of the lens. Cx46 mutations are associated with impairment of channel function, which results in the development of congenital cataracts. Cx46 gap junctions and hemichannels are closely regulated by multiple mechanisms. Key regulators of Cx46 channel function include Ca2+ and calmodulin (CaM). Ca2+ plays an essential role in lens homeostasis, and its dysregulation causes cataracts. Ca2+ associated CaM is a well-established inhibitor of gap junction coupling. Recent studies suggest that elevated intracellular Ca2+ activates Cx hemichannels in lens fiber cells and Cx46 directly interacts with CaM. A Cx46 site mutation (Cx46-G143R), which is associated with congenital Coppock cataracts, shows an increased Cx46-CaM interaction and this interaction is insensitive to Ca2+, given that depletion of Ca2+ reduces the interaction between CaM and wild-type Cx46. Moreover, inhibition of CaM function greatly reduces the hemichannel activity in the Cx46 G143R mutant. These research findings suggest a new regulatory mechanism by which enhanced association of Cx46 with CaM leads to the increase in hemichannel activity and dysregulation may lead to cataract development. In this review, we will first discuss the involvement of Ca2+/CaM in lens homeostasis and pathology, and follow by providing a general overview of Ca2+/CaM in the regulation of Cx46 gap junctions. We discuss the most recent studies concerning the molecular mechanism of Ca2+/CaM in regulating Cx46 hemichannels. Finally, we will offer perspectives of the impacts of Ca2+/CaM and dysregulation on Cx46 channels and vice versa.
Keywords: calcium; calmodulin; connexin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

