Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation
- PMID: 33147748
- PMCID: PMC7692386
- DOI: 10.3390/nu12113373
Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation
Abstract
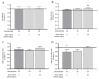
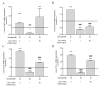
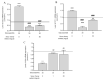
Diabetes mellitus (DM)-related morbidity and mortality are steadily rising worldwide, affecting about half a billion people worldwide. A significant proportion of diabetic cases are in the elderly, which is concerning given the increasing aging population. Proper nutrition is an important component in the effective management of diabetes in the elderly. A plethora of active substances of plant origin exhibit potency to target the pathogenesis of diabetes mellitus. The nutraceutical and pharmaceutical effects of anthocyanins have been extensively studied. In this study, the effect of Hungarian sour cherry, which is rich in anthocyanins, on hyperglycemia-induced endothelial dysfunction was tested using human umbilical cord vein endothelial cells (HUVECs). HUVECs were maintained under both normoglycemic (5 mM) and hyperglycemic (30 mM) conditions with or without two concentrations (1.50 ng/µL) of anthocyanin-rich sour cherry extract. Hyperglycemia-induced oxidative stress and inflammatory response and damaged vasorelaxation processes were investigated by evaluating the level of reactive oxygen species (ROS) and gene expression of four proinflammatory cytokines, namely, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-8 (IL-8), and interleukin-1α (IL-1α), as well as the gene expression of nitric oxide synthase (NOS) endothelin-1 (ET-1) and endothelin-converting enzyme-1 (ECE-1). It was found that hyperglycemia-induced oxidative stress was significantly suppressed by anthocyanin-rich sour cherry extract in a concentration-dependent manner. The gene expression of the tested proinflammatory cytokines increased under hyperglycemic conditions but was significantly reduced by both 1 and 50 ng/µL anthocyanin-rich sour cherry extract. Further, although increased ET-1 and ECE-1 expression due to hyperglycemia was reduced by anthocyanin-rich sour cherry extract, NOS expression was increased by the extract. Collectively, these data suggest that anthocyanin-rich sour cherry extract could alleviate hyperglycemia-induced endothelial dysfunction due to its antioxidant, anti-inflammatory, and vasorelaxant effects.
Keywords: anthocyanins; endothelial dysfunction; hyperglycemia; vasodilation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Director-General Addresses US Department of Health and Human Services. [(accessed on 25 August 2020)]; Available online: https://www.who.int/dg/speeches/detail/who-director-general-addresses-us....
-
- International Diabetes Federation—Facts & Figures. [(accessed on 25 August 2020)]; Available online: https://idf.org/aboutdiabetes/what-is-diabetes/factsfigures.html?fbclid=....
-
- Tan S.Y., Mei Wong J.L., Sim Y.J., Wong S.S., Mohamed Elhassan S.A., Tan S.H., Ling Lim G.P., Rong Tay N.W., Annan N.C., Bhattamisra S.K., et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019;13:364–372. doi: 10.1016/j.dsx.2018.10.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

