New Methods Used in Pharmacokinetics and Therapeutic Monitoring of the First and Newer Generations of Antiepileptic Drugs (AEDs)
- PMID: 33147810
- PMCID: PMC7663638
- DOI: 10.3390/molecules25215083
New Methods Used in Pharmacokinetics and Therapeutic Monitoring of the First and Newer Generations of Antiepileptic Drugs (AEDs)
Abstract
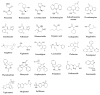
The review presents data from the last few years on bioanalytical methods used in therapeutic drug monitoring (TDM) of the 1st-3rd generation and the newest antiepileptic drug (AEDs) cenobamate in patients with various forms of seizures. Chemical classification, structure, mechanism of action, pharmacokinetic data and therapeutic ranges for total and free fractions and interactions were collected. The primary data on bioanalytical methods for AEDs determination included biological matrices, sample preparation, dried blood spot (DBS) analysis, column resolution, detection method, validation parameters, and clinical utility. In conclusion, the most frequently described method used in AED analysis is the LC-based technique (HPLC, UHPLC, USLC) combined with highly sensitive mass detection or fluorescence detection. However, less sensitive UV is also used. Capillary electrophoresis and gas chromatography have been rarely applied. Besides the precipitation of proteins or LLE, an automatic SPE is often a sample preparation method. Derivatization was also indicated to improve sensitivity and automate the analysis. The usefulness of the methods for TDM was also highlighted.
Keywords: antiepileptic drugs; bioanalysis; capillary electrophoresis; clinical samples; dried blood spots; high-performance liquid chromatography; pharmacokinetics; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cenobamate (Xcopri) for focal seizures.Med Lett Drugs Ther. 2020 Aug 24;62(1605):134-136. Med Lett Drugs Ther. 2020. PMID: 32970044 Review. No abstract available.
-
Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed?Clin Pharmacokinet. 2006;45(11):1061-75. doi: 10.2165/00003088-200645110-00002. Clin Pharmacokinet. 2006. PMID: 17048972 Review.
-
Dried blood spots for monitoring and individualization of antiepileptic drug treatment.Eur J Pharm Sci. 2015 Jul 30;75:25-39. doi: 10.1016/j.ejps.2015.04.008. Epub 2015 Apr 17. Eur J Pharm Sci. 2015. PMID: 25896371 Review.
-
LC-MS/MS-Based Quantification of 9 Antiepileptic Drugs From a Dried Sample Spot Device.Ther Drug Monit. 2019 Jun;41(3):331-339. doi: 10.1097/FTD.0000000000000600. Ther Drug Monit. 2019. PMID: 30688867
-
Pharmacology of Cenobamate: Mechanism of Action, Pharmacokinetics, Drug-Drug Interactions and Tolerability.CNS Drugs. 2021 Jun;35(6):609-618. doi: 10.1007/s40263-021-00819-8. Epub 2021 May 16. CNS Drugs. 2021. PMID: 33993416 Review.
Cited by
-
Evaluating the efficacy of prototype antiseizure drugs using a preclinical pharmacokinetic approach.Epilepsia. 2022 Nov;63(11):2937-2948. doi: 10.1111/epi.17402. Epub 2022 Sep 11. Epilepsia. 2022. PMID: 36054499 Free PMC article.
-
The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond.CNS Drugs. 2021 Sep;35(9):935-963. doi: 10.1007/s40263-021-00827-8. Epub 2021 Jun 18. CNS Drugs. 2021. PMID: 34145528 Free PMC article. Review.
-
Role of Chalcogenides in Sensitive Therapeutic Drug Monitoring Using Laser Desorption and Ionization.ACS Nano. 2024 Jul 9;18(27):17681-17693. doi: 10.1021/acsnano.4c02429. Epub 2024 Jun 26. ACS Nano. 2024. PMID: 38920103 Free PMC article.
-
Pathophysiology to Risk Factor and Therapeutics to Treatment Strategies on Epilepsy.Brain Sci. 2024 Jan 10;14(1):71. doi: 10.3390/brainsci14010071. Brain Sci. 2024. PMID: 38248286 Free PMC article. Review.
-
Development and Validation of a UHPLC-MS/MS-Based Method to Quantify Cenobamate in Human Plasma Samples.Molecules. 2022 Oct 28;27(21):7325. doi: 10.3390/molecules27217325. Molecules. 2022. PMID: 36364153 Free PMC article.
References
-
- XCOPRI. Full Prescribing Information. [(accessed on 13 July 2020)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212839s000lbl.pdf.
-
- Cenobamate (XCOPRI). Clinical Pharmacology and Biopharmaceutics Review(s); Application Number 212839Orig1s000. [(accessed on 13 July 2020)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212839Orig1s000C....
-
- Cusumano J.A., Klinker K.P., Huttner A., Luther M.K., Roberts J.A., LaPlante K.L. Towards precision medicine: Therapeutic drug monitoring–guided dosing of vancomycin and β-lactam antibiotics to maximize effectiveness and minimize toxicity. Am. J. Health Syst. Pharm. 2020;77:1104–1112. doi: 10.1093/ajhp/zxaa128. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical