Inhibition of Human Neutrophil Functions In Vitro by Multiple Sclerosis Disease-Modifying Therapies
- PMID: 33147889
- PMCID: PMC7692529
- DOI: 10.3390/jcm9113542
Inhibition of Human Neutrophil Functions In Vitro by Multiple Sclerosis Disease-Modifying Therapies
Abstract
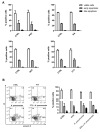
There is a growing optimism about the potential of new disease-modifying therapies (DMTs) in the management of relapsing-remitting multiple sclerosis (RRMS) patients. However, this initial enthusiasm has been tempered by evidence indicating that multiple sclerosis (MS) patients undergoing DMT may be at higher risk of developing infections through incompletely understood mechanisms. As neutrophils provide the first line of defense against pathogens, here we have compared the effects of some of the commonly used MS DMTs (i.e., moderate-efficacy injective, first-line: interferonβ-1b (IFNβ-1b), glatiramer acetate (GA); and high-efficacy, second-line: fingolimod (FTY) and natalizumab (NAT)) on the in vitro viability and functions of neutrophils isolated from healthy subjects. All the DMTs tested impaired the ability of neutrophils to kill Klebsiella pneumoniae, whereas none of them affected the rate of neutrophil apoptosis or CD11b and CD62L cell surface expression. Intriguingly, only FTY exposure negatively affected K. pneumoniae-induced production of reactive oxygen species (ROS) in polymorphonuclear leukocytes (PMNs). Furthermore, neutrophils exposed to K. pneumoniae secreted enhanced amounts of CXCL8, IL-1β and TNF-α, which were differentially regulated following DMT pretreatment. Altogether, these findings suggest that DMTs may increase the susceptibility of MS patients to microbial infections, in part, through inhibition of neutrophil functions. In light of these data, we recommend that the design of personalized therapies for RRMS patients should take into account not just the mechanism of action of the chosen DMT but also the potential risk of infection associated with the administration of such therapeutic compounds to this highly vulnerable population.
Keywords: Klebsiella pneumoniae; disease-modifying therapies; multiple sclerosis; neutrophil functions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Luna G., Alping P., Burman J., Fink K., Fogdell-Hahn A., Gunnarsson M., Hillert J., Langer-Gould A., Lycke J., Nilsson P., et al. Infection Risks Among Patients with Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2020;77:184–191. doi: 10.1001/jamaneurol.2019.3365. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

