APOE2: protective mechanism and therapeutic implications for Alzheimer's disease
- PMID: 33148290
- PMCID: PMC7640652
- DOI: 10.1186/s13024-020-00413-4
APOE2: protective mechanism and therapeutic implications for Alzheimer's disease
Abstract
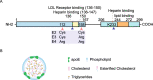
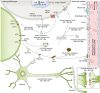
Investigations of apolipoprotein E (APOE) gene, the major genetic risk modifier for Alzheimer's disease (AD), have yielded significant insights into the pathogenic mechanism. Among the three common coding variants, APOE*ε4 increases, whereas APOE*ε2 decreases the risk of late-onset AD compared with APOE*ε3. Despite increased understanding of the detrimental effect of APOE*ε4, it remains unclear how APOE*ε2 confers protection against AD. Accumulating evidence suggests that APOE*ε2 protects against AD through both amyloid-β (Aβ)-dependent and independent mechanisms. In addition, APOE*ε2 has been identified as a longevity gene, suggesting a systemic effect of APOE*ε2 on the aging process. However, APOE*ε2 is not entirely benign; APOE*ε2 carriers exhibit increased risk of certain cerebrovascular diseases and neurological disorders. Here, we review evidence from both human and animal studies demonstrating the protective effect of APOE*ε2 against AD and propose a working model depicting potential underlying mechanisms. Finally, we discuss potential therapeutic strategies designed to leverage the protective effect of APOE2 to treat AD.
Keywords: Alzheimer’s disease; Amyloid-β; Apolipoprotein E2; Cerebrovascular disease; Lipid metabolism; Longevity; Neurofibrially tangles; Neuroinflammation; TDP-43; α-Synuclein.
Conflict of interest statement
The authors declared no competing of interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

