Changes in DNA methylation profiles of myalgic encephalomyelitis/chronic fatigue syndrome patients reflect systemic dysfunctions
- PMID: 33148325
- PMCID: PMC7641803
- DOI: 10.1186/s13148-020-00960-z
Changes in DNA methylation profiles of myalgic encephalomyelitis/chronic fatigue syndrome patients reflect systemic dysfunctions
Abstract
Background: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a lifelong debilitating disease with a complex pathology not yet clearly defined. Susceptibility to ME/CFS involves genetic predisposition and exposure to environmental factors, suggesting an epigenetic association. Epigenetic studies with other ME/CFS cohorts have used array-based technology to identify differentially methylated individual sites. Changes in RNA quantities and protein abundance have been documented in our previous investigations with the same ME/CFS cohort used for this study.
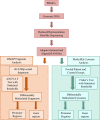
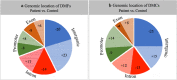
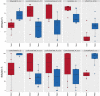
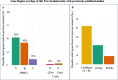
Results: DNA from a well-characterised New Zealand cohort of 10 ME/CFS patients and 10 age-/sex-matched healthy controls was isolated from peripheral blood mononuclear (PBMC) cells, and used to generate reduced genome-scale DNA methylation maps using reduced representation bisulphite sequencing (RRBS). The sequencing data were analysed utilising the DMAP analysis pipeline to identify differentially methylated fragments, and the MethylKit pipeline was used to quantify methylation differences at individual CpG sites. DMAP identified 76 differentially methylated fragments and Methylkit identified 394 differentially methylated cytosines that included both hyper- and hypo-methylation. Four clusters were identified where differentially methylated DNA fragments overlapped with or were within close proximity to multiple differentially methylated individual cytosines. These clusters identified regulatory regions for 17 protein encoding genes related to metabolic and immune activity. Analysis of differentially methylated gene bodies (exons/introns) identified 122 unique genes. Comparison with other studies on PBMCs from ME/CFS patients and controls with array technology showed 59% of the genes identified in this study were also found in one or more of these studies. Functional pathway enrichment analysis identified 30 associated pathways. These included immune, metabolic and neurological-related functions differentially regulated in ME/CFS patients compared to the matched healthy controls.
Conclusions: Major differences were identified in the DNA methylation patterns of ME/CFS patients that clearly distinguished them from the healthy controls. Over half found in gene bodies with RRBS in this study had been identified in other ME/CFS studies using the same cells but with array technology. Within the enriched functional immune, metabolic and neurological pathways, a number of enriched neurotransmitter and neuropeptide reactome pathways highlighted a disturbed neurological pathophysiology within the patient group.
Keywords: DMAP; DNA methylation; Epigenetics; ME/CFS; MethylKit; RRBS.
Conflict of interest statement
The authors declare they have no competing interests.
Figures


References
-
- Shepherd C. ME/CFS/PVFS: an exploration of the key clinical issues. Gawcott: ME Association; 2013. p. 52.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

