Pneumococcal serotypes in children, clinical presentation and antimicrobial susceptibility in the PCV13 era
- PMID: 33148361
- PMCID: PMC7770381
- DOI: 10.1017/S0950268820002708
Pneumococcal serotypes in children, clinical presentation and antimicrobial susceptibility in the PCV13 era
Abstract
The aim was to analyse invasive pneumococcal disease (IPD) serotypes in children aged ⩽17 years according to clinical presentation and antimicrobial susceptibility. We conducted a prospective study (January 2012-June 2016). IPD cases were diagnosed by culture and/or real-time polymerase chain reaction (PCR). Demographic, microbiological and clinical data were analysed. Associations were assessed using the odds ratio (OR) and 95% confidence intervals (CI). Of the 253 cases, 34.4% were aged <2 years, 38.7% 2-4 years and 26.9% 5-17 years. Over 64% were 13-valent pneumococcal conjugate vaccine (PCV13) serotypes. 48% of the cases were diagnosed only by real-time PCR. Serotypes 3 and 1 were associated with complicated pneumonia (P < 0.05) and non-PCV13 serotypes with meningitis (OR 7.32, 95% CI 2.33-22.99) and occult bacteraemia (OR 3.6, 95% CI 1.56-8.76). Serotype 19A was more frequent in children aged <2 years and serotypes 3 and 1 in children aged 2-4 years and 5-17 years, respectively. 36.1% of cases were not susceptible to penicillin and 16.4% were also non-susceptible to cefotaxime. Serotypes 14, 24F and 23B were associated with non-susceptibility to penicillin (P < 0.05) and serotypes 11, 14 and 19A to cefotaxime (P < 0.05). Serotype 19A showed resistance to penicillin (P = 0.002). In conclusion, PCV13 serotypes were most frequent in children aged ⩽17 years, mainly serotypes 3, 1 and 19A. Non-PCV13 serotypes were associated with meningitis and occult bacteraemia and PCV13 serotypes with pneumonia. Non-susceptibility to antibiotics of non-PCV13 serotypes should be monitored.
Keywords: Antimicrobial susceptibility; PCV13 vaccine; Streptococcus pneumoniae; invasive pneumococcal disease; penicillin resistance; serotype distribution.
Conflict of interest statement
JJ Garcia-Garcia has received honoraria for speaking at symposia (Pfizer and GSK), and financial support for attending symposia (Pfizer). None were concerned with the present study.
C Muñoz-Almagro reports travel grants from Pfizer, research grants from BioMerieux, Stat DX and Instituto de Salud Carlos III, personal fees from GSK as consultor to an advisory board and honoraria for speaking at symposia from Roche and Biomerieux. None were concerned with the present study.
For the remaining authors, no competing interests were declared.
Figures

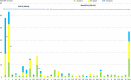

<2 age group. #1: P < 0.001; #2: P = 0.004 (non-complicated and complicated pneumonia); #3:P = 0.033.
2–4 age group. #4: P = 0.002; #5:P = 0.003
5–17 age group. #6:P < 0.001; #7: P = 0.004; #8: P = 0.004
References
-
- Whitney CG et al. (2003) Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. The New England Journal of Medicine 348, 1737–1746. - PubMed
-
- Rückinger S et al. (2009) Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine 27, 4136–4141. - PubMed
-
- Harboe ZB et al. (2010) Early effectiveness of heptavalent conjugate pneumococcal vaccination on invasive pneumococcal disease after the introduction in the Danish childhood immunization programme. Vaccine 28, 2642–2647. - PubMed
-
- Roche PW et al. (2008) Invasive pneumococcal disease in Australia, 2006. Communicable Diseases Intelligence 32, 18–30. - PubMed

