Neutrophil-Macrophage Imbalance Drives the Development of Renal Scarring during Experimental Pyelonephritis
- PMID: 33148615
- PMCID: PMC7894670
- DOI: 10.1681/ASN.2020030362
Neutrophil-Macrophage Imbalance Drives the Development of Renal Scarring during Experimental Pyelonephritis
Abstract
Background: In children, the acute pyelonephritis that can result from urinary tract infections (UTIs), which commonly ascend from the bladder to the kidney, is a growing concern because it poses a risk of renal scarring and irreversible loss of kidney function. To date, the cellular mechanisms underlying acute pyelonephritis-driven renal scarring remain unknown.
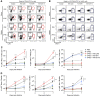
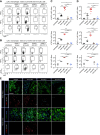
Methods: We used a preclinical model of uropathogenic Escherichia coli-induced acute pyelonephritis to determine the contribution of neutrophils and monocytes to resolution of the condition and the subsequent development of kidney fibrosis. We used cell-specific monoclonal antibodies to eliminate neutrophils, monocytes, or both. Bacterial ascent and the cell dynamics of phagocytic cells were assessed by biophotonic imaging and flow cytometry, respectively. We used quantitative RT-PCR and histopathologic analyses to evaluate inflammation and renal scarring.
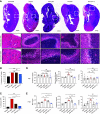
Results: We found that neutrophils are critical to control bacterial ascent, which is in line with previous studies suggesting a protective role for neutrophils during a UTI, whereas monocyte-derived macrophages orchestrate a strong, but ineffective, inflammatory response against uropathogenic, E. coli-induced, acute pyelonephritis. Experimental neutropenia during acute pyelonephritis resulted in a compensatory increase in the number of monocytes and heightened macrophage-dependent inflammation in the kidney. Exacerbated macrophage-mediated inflammatory responses promoted renal scarring and compromised renal function, as indicated by elevated serum creatinine, BUN, and potassium.
Conclusions: These findings reveal a previously unappreciated outcome for neutrophil-macrophage imbalance in promoting host susceptibility to acute pyelonephritis and the development of permanent renal damage. This suggests targeting dysregulated macrophage responses might be a therapeutic tool to prevent renal scarring during acute pyelonephritis.
Keywords: acute pyelonephritis; inflammation; macrophages; neutrophils; renal scarring; urinary tract infection.
Copyright © 2021 by the American Society of Nephrology.
Figures






Similar articles
-
Androgen-Influenced Polarization of Activin A-Producing Macrophages Accompanies Post-pyelonephritic Renal Scarring.Front Immunol. 2020 Jul 28;11:1641. doi: 10.3389/fimmu.2020.01641. eCollection 2020. Front Immunol. 2020. PMID: 32849562 Free PMC article.
-
Inflammation drives renal scarring in experimental pyelonephritis.Am J Physiol Renal Physiol. 2017 Jan 1;312(1):F43-F53. doi: 10.1152/ajprenal.00471.2016. Epub 2016 Oct 19. Am J Physiol Renal Physiol. 2017. PMID: 27760770 Free PMC article.
-
Androgen exposure impairs neutrophil maturation and function within the infected kidney.mBio. 2024 Feb 14;15(2):e0317023. doi: 10.1128/mbio.03170-23. Epub 2024 Jan 11. mBio. 2024. PMID: 38206009 Free PMC article.
-
Dimercaptosuccinic acid renal scintigraphy for the evaluation of pyelonephritis and scarring: a review of experimental and clinical studies.J Urol. 1992 Nov;148(5 Pt 2):1726-32. doi: 10.1016/s0022-5347(17)37014-3. J Urol. 1992. PMID: 1331545 Review.
-
Bacterial attachment, inflammation and renal scarring in urinary tract infection.Wien Med Wochenschr. 1991;141(23-24):537-40. Wien Med Wochenschr. 1991. PMID: 1810092 Review.
Cited by
-
Kidney immunology from pathophysiology to clinical translation.Nat Rev Immunol. 2025 Jun;25(6):460-476. doi: 10.1038/s41577-025-01131-y. Epub 2025 Jan 30. Nat Rev Immunol. 2025. PMID: 39885266 Review.
-
Uropathogen and host responses in pyelonephritis.Nat Rev Nephrol. 2023 Oct;19(10):658-671. doi: 10.1038/s41581-023-00737-6. Epub 2023 Jul 21. Nat Rev Nephrol. 2023. PMID: 37479904 Free PMC article. Review.
-
Murine Ribonuclease 6 Limits Bacterial Dissemination during Experimental Urinary Tract Infection.J Innate Immun. 2024;16(1):283-294. doi: 10.1159/000539177. Epub 2024 May 14. J Innate Immun. 2024. PMID: 38744252 Free PMC article.
-
Immune defenses in the urinary tract.Trends Immunol. 2023 Sep;44(9):701-711. doi: 10.1016/j.it.2023.07.001. Epub 2023 Aug 15. Trends Immunol. 2023. PMID: 37591712 Free PMC article. Review.
-
Effect of anticoagulant and platelet inhibition on the risk of bacteremia among patients with acute pyelonephritis: a retrospective cohort study.BMC Infect Dis. 2022 May 31;22(1):509. doi: 10.1186/s12879-022-07474-4. BMC Infect Dis. 2022. PMID: 35641940 Free PMC article.
References
-
- Freedman AL; Urologic Diseases in America Project: Urologic diseases in North America Project: Trends in resource utilization for urinary tract infections in children. J Urol 173: 949–954, 2005 - PubMed
-
- Shaikh N, Ewing AL, Bhatnagar S, Hoberman A: Risk of renal scarring in children with a first urinary tract infection: A systematic review. Pediatrics 126: 1084–1091, 2010 - PubMed
-
- Shaikh N, Craig JC, Rovers MM, Da Dalt L, Gardikis S, Hoberman A, et al. .: Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: A meta-analysis with individual patient data. JAMA Pediatr 168: 893–900, 2014 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

