Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice
- PMID: 33148622
- PMCID: PMC7886371
- DOI: 10.1126/scitranslmed.aba0599
Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice
Abstract
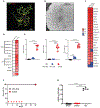
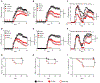
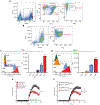
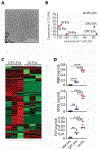
Autoimmune diseases such as multiple sclerosis (MS) develop because of failed peripheral immune tolerance for a specific self-antigen (Ag). Numerous approaches for Ag-specific suppression of autoimmune neuroinflammation have been proven effective in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. One such approach is intravenous tolerance induction by injecting a myelin Ag used for triggering EAE. However, the translation of this and similar experimental strategies into therapy for MS has been hampered by uncertainty regarding relevant myelin Ags in MS patients. To address this issue, we developed a therapeutic strategy that relies on oligodendrocyte (Ol)-derived extracellular vesicles (Ol-EVs), which naturally contain multiple myelin Ags. Intravenous Ol-EV injection reduced disease pathophysiology in a myelin Ag-dependent manner, both prophylactically and therapeutically, in several EAE models. The treatment was safe and restored immune tolerance by inducing immunosuppressive monocytes and apoptosis of autoreactive CD4+ T cells. Furthermore, we showed that human Ols also released EVs containing most relevant myelin Ags, providing a basis for their use in MS therapy. These findings introduce an approach for suppressing central nervous system (CNS) autoimmunity in a myelin Ag-specific manner, without the need to identify the target Ag.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



Similar articles
-
Clinical and immunological control of experimental autoimmune encephalomyelitis by tolerogenic dendritic cells loaded with MOG-encoding mRNA.J Neuroinflammation. 2019 Aug 15;16(1):167. doi: 10.1186/s12974-019-1541-1. J Neuroinflammation. 2019. PMID: 31416452 Free PMC article.
-
Interferon-γ/Interleukin-27 Axis Induces Programmed Death Ligand 1 Expression in Monocyte-Derived Dendritic Cells and Restores Immune Tolerance in Central Nervous System Autoimmunity.Front Immunol. 2020 Oct 26;11:576752. doi: 10.3389/fimmu.2020.576752. eCollection 2020. Front Immunol. 2020. PMID: 33193372 Free PMC article.
-
A Tolerogenic Artificial APC Durably Ameliorates Experimental Autoimmune Encephalomyelitis by Directly and Selectively Modulating Myelin Peptide-Autoreactive CD4+ and CD8+ T Cells.J Immunol. 2018 Aug 15;201(4):1194-1210. doi: 10.4049/jimmunol.1800108. Epub 2018 Jul 9. J Immunol. 2018. PMID: 29987163
-
T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis.Glia. 2001 Nov;36(2):220-34. doi: 10.1002/glia.1111. Glia. 2001. PMID: 11596130 Review.
-
The role of myelin oligodendrocyte glycoprotein in autoimmune demyelination: a target for multiple sclerosis therapy?Expert Opin Ther Targets. 2012 May;16(5):451-62. doi: 10.1517/14728222.2012.677438. Epub 2012 Apr 12. Expert Opin Ther Targets. 2012. PMID: 22494461 Review.
Cited by
-
PEGylation enables subcutaneously administered nanoparticles to induce antigen-specific immune tolerance.J Control Release. 2021 Mar 10;331:164-175. doi: 10.1016/j.jconrel.2021.01.013. Epub 2021 Jan 12. J Control Release. 2021. PMID: 33450320 Free PMC article.
-
Antigen-specific therapy for multiple sclerosis.Nat Rev Drug Discov. 2021 Jan;20(1):20. doi: 10.1038/d41573-020-00211-z. Nat Rev Drug Discov. 2021. PMID: 33262510 No abstract available.
-
The influence of the gut microbiota on B cells in autoimmune diseases.Mol Med. 2025 Apr 22;31(1):149. doi: 10.1186/s10020-025-01195-5. Mol Med. 2025. PMID: 40264032 Free PMC article. Review.
-
Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders.Signal Transduct Target Ther. 2024 Oct 4;9(1):263. doi: 10.1038/s41392-024-01952-8. Signal Transduct Target Ther. 2024. PMID: 39362875 Free PMC article. Review.
-
Extracellular Vesicles in Mental Disorders: A State-of-art Review.Int J Biol Sci. 2023 Feb 5;19(4):1094-1109. doi: 10.7150/ijbs.79666. eCollection 2023. Int J Biol Sci. 2023. PMID: 36923936 Free PMC article. Review.
References
-
- Faissner S, Plemel JR, Gold R, Yong VW, Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov 18, 905–922 (2019). - PubMed
-
- Steinman L, Ho PP, Robinson WH, Utz PJ, Villoslada P, Antigen-specific tolerance to self-antigens in protein replacement therapy, gene therapy and autoimmunity. Curr. Opin. Immunol 61, 46–53 (2019). - PubMed
-
- Serra P, Santamaria P, Antigen-specific therapeutic approaches for autoimmunity. Nat. Biotechnol 37, 238–251 (2019). - PubMed
-
- Zwhang G-X, Yu S, Calida D, Zhao Z, Gran B, Kamoun M, Rostami A, Loss of the surface antigen 3G11 characterizes a distinct population of anergic/regulatory T cells in experimental autoimmune encephalomyelitis. J. Immunol 176, 3366–3373 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

