Switching between Janus kinase inhibitor upadacitinib and adalimumab following insufficient response: efficacy and safety in patients with rheumatoid arthritis
- PMID: 33148701
- PMCID: PMC7958110
- DOI: 10.1136/annrheumdis-2020-218412
Switching between Janus kinase inhibitor upadacitinib and adalimumab following insufficient response: efficacy and safety in patients with rheumatoid arthritis
Abstract
Objectives: To evaluate efficacy and safety of immediate switch from upadacitinib to adalimumab, or vice versa, in patients with rheumatoid arthritis with non-response or incomplete-response to the initial therapy.
Methods: SELECT-COMPARE randomised patients to upadacitinib 15 mg once daily (n=651), placebo (n=651) or adalimumab 40 mg every other week (n=327). A treat-to-target study design was implemented, with blinded rescue occurring prior to week 26 for patients who did not achieve at least 20% improvement in both tender and swollen joint counts ('non-responders') and at week 26 based on Clinical Disease Activity Index (CDAI) >10 ('incomplete-responders') without washout.
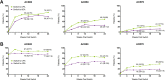
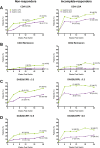
Results: A total of 39% (252/651) and 49% (159/327) of patients originally randomised to upadacitinib and adalimumab were rescued to the alternate therapy. In both switch groups (adalimumab to upadacitinib and vice versa) and in non-responders and incomplete-responders, improvements in disease activity were observed at 3 and 6 months following rescue. CDAI low disease activity was achieved by 36% and 47% of non-responders and 45% and 58% of incomplete-responders switched to adalimumab and upadacitinib, respectively, 6 months following switch. Overall, approximately 5% of rescued patients experienced worsening in disease activity at 6 months postswitch. The frequency of adverse events was similar between switch groups.
Conclusions: These observations support a treat-to-target strategy, in which patients who fail to respond initially (or do not achieve sufficient response) are switched to a therapy with an alternate mechanism of action and experience improved outcomes. No new safety findings were observed despite immediate switch without washout.
Trial registration: ClinicalTrials.gov NCT02629159.
Keywords: adalimumab; arthritis; health care; outcome assessment; rheumatoid; therapeutics; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RMF has received grant/research support from AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD-Serono, Genentech, Gilead, Janssen, Novartis, Pfizer Inc, Regeneron, Roche, Sanofi-Aventis, UCB and Viela and is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer Inc, Sanofi-Aventis and UCB. RB has received grants/research support from AbbVie, MSD and Roche and had consultation fees/participation in company-sponsored speaker’s bureau from AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Pfizer, and Roche. SH has received research grants and consultancy fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer and UCB. GTDT has received a research grant from AbbVie and consulting fees from Amgen. FEVdB has received speaker and/or consultancy fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer and UCB. CZ has received grants/research support from AbbVie, Amgen, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer and Sanofi. LB has received grant/research support from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer Inc, Sanofi-Aventis and UCB and is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Gilead, Janssen, Novartis, Pfizer Inc, Sanofi-Aventis and UCB. JE, YL, YS, RD and I-HS are full-time employees of AbbVie and may hold AbbVie stock or stock options.
Figures