Chikungunya Virus Replication Rate Determines the Capacity of Crossing Tissue Barriers in Mosquitoes
- PMID: 33148794
- PMCID: PMC7925089
- DOI: 10.1128/JVI.01956-20
Chikungunya Virus Replication Rate Determines the Capacity of Crossing Tissue Barriers in Mosquitoes
Abstract
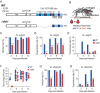
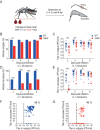
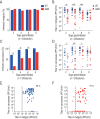
Chikungunya virus (CHIKV) is a reemerging and rapidly spreading pathogen transmitted by mosquitoes. The emergence of new epidemic variants of the virus is associated with genetic evolutionary traits, including duplication of repeated RNA elements in the 3' untranslated region (UTR) that seemingly favor transmission by mosquitoes. The transmission potential of a given variant results from a complex interplay between virus populations and anatomical tissue barriers in the mosquito. Here, we used the wild-type CHIKV Caribbean strain and an engineered mutant harboring a deletion in the 3' UTR to dissect the interactions of virus variants with the anatomical barriers that impede transmission during the replication cycle of the virus in Aedes mosquitoes. Compared to the 3'-UTR mutant, we observed that the wild-type virus had a short extrinsic incubation period (EIP) after an infectious blood meal and was expectorated into mosquito saliva much more efficiently. We found that high viral titers in the midgut are not sufficient to escape the midgut escape barrier. Rather, viral replication kinetics play a crucial role in determining midgut escape and the transmission ability of CHIKV. Finally, competition tests in mosquitoes coinfected with wild-type and mutant viruses revealed that both viruses successfully colonized the midgut, but wild-type viruses effectively displaced mutant viruses during systemic infection due to their greater efficiency of escaping from the midgut into secondary tissues. Overall, our results uncover a link between CHIKV replication kinetics and the effect of bottlenecks on population diversity, as slowly replicating variants are less able to overcome the midgut escape barrier.IMPORTANCE It is well established that selective pressures in mosquito vectors impose population bottlenecks for arboviruses. Here, we used a CHIKV Caribbean lineage mutant carrying a deletion in the 3' UTR to study host-virus interactions in vivo in the epidemic mosquito vector Aedes aegypti We found that the mutant virus had a delayed replication rate in mosquitoes, which lengthened the extrinsic incubation period (EIP) and reduced fitness relative to the wild-type virus. As a result, the mutant virus displayed a reduced capacity to cross anatomical barriers during the infection cycle in mosquitoes, thus reducing the virus transmission rate. Our findings show how selective pressures act on CHIKV noncoding regions to select variants with shorter EIPs that are preferentially transmitted by the mosquito vector.
Keywords: 3′ UTR; alphavirus; arthropod vectors; bottlenecks; extrinsic incubation period.
Copyright © 2021 American Society for Microbiology.
Figures



References
-
- Jones R, Kulkarni MA, Davidson TMV, RADAM-LAC Research Team, Talbot B. 2020. Arbovirus vectors of epidemiological concern in the Americas: a scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS One 15:e0220753. doi:10.1371/journal.pone.0220753. - DOI - PMC - PubMed
-
- Thaikruea L, Charearnsook O, Reanphumkarnkit S, Dissomboon P, Phonjan R, Ratchbud S, Kounsang Y, Buranapiyawong D. 1997. Chikungunya in Thailand: a re-emerging disease? Southeast Asian J Trop Med Public Health 28:359–364. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

