Implications of Integrated Pancreatic Microcirculation: Crosstalk between Endocrine and Exocrine Compartments
- PMID: 33148810
- PMCID: PMC7679783
- DOI: 10.2337/db20-0810
Implications of Integrated Pancreatic Microcirculation: Crosstalk between Endocrine and Exocrine Compartments
Abstract
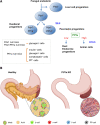
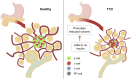
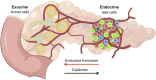
The endocrine and exocrine pancreas have been studied separately by endocrinologists and gastroenterologists as two organ systems. The pancreatic islet, consisting of 1-2% mass of the whole pancreas, has long been believed to be regulated independently from the surrounding exocrine tissues. Particularly, islet blood flow has been consistently illustrated as one-way flow from arteriole(s) to venule(s) with no integration of the capillary network between the endocrine and exocrine pancreas. It is likely linked to the long-standing dogma that the rodent islet has a mantle of non-β-cells and that the islet is completely separated from the exocrine compartment. A new model of islet microcirculation is built on the basis of analyses of in vivo blood flow measurements in mice and an in situ three-dimensional structure of the capillary network in mice and humans. The deduced integrated blood flow throughout the entire pancreas suggests direct interactions between islet endocrine cells and surrounding cells as well as the bidirectional blood flow between the endocrine and exocrine pancreas, not necessarily a unidirectional blood flow as in a so-called insuloacinar portal system. In this perspective, we discuss how this conceptual transformation could potentially affect our current understanding of the biology, physiology, and pathogenesis of the islet and pancreas.
© 2020 by the American Diabetes Association.
Figures


Similar articles
-
Integrated Pancreatic Blood Flow: Bidirectional Microcirculation Between Endocrine and Exocrine Pancreas.Diabetes. 2020 Jul;69(7):1439-1450. doi: 10.2337/db19-1034. Epub 2020 Mar 20. Diabetes. 2020. PMID: 32198213 Free PMC article.
-
In Vivo and In Situ Approach to Study Islet Microcirculation: A Mini-Review.Front Endocrinol (Lausanne). 2021 May 10;12:602620. doi: 10.3389/fendo.2021.602620. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34040578 Free PMC article. Review.
-
Local Dialogues Between the Endocrine and Exocrine Cells in the Pancreas.Diabetes. 2024 Apr 1;73(4):533-541. doi: 10.2337/db23-0760. Diabetes. 2024. PMID: 38215069 Free PMC article. Review.
-
Islet vasculature as a regulator of endocrine pancreas function.World J Surg. 2007 Apr;31(4):705-14. doi: 10.1007/s00268-006-0719-8. World J Surg. 2007. PMID: 17347899 Review.
-
[Light and scanning electron microscopic study on portal circulation of the pancreas in the monkey].Zhonghua Yi Xue Za Zhi. 1991 Sep;71(9):485-7, 34. Zhonghua Yi Xue Za Zhi. 1991. PMID: 1660772 Chinese.
Cited by
-
Role of insulin in pancreatic microcirculatory oxygen profile and bioenergetics.World J Diabetes. 2022 Sep 15;13(9):765-775. doi: 10.4239/wjd.v13.i9.765. World J Diabetes. 2022. PMID: 36188151 Free PMC article.
-
Integrated pancreatic microcirculatory profiles of streptozotocin-induced and insulin-administrated type 1 diabetes mellitus.Microcirculation. 2021 Jul;28(5):e12691. doi: 10.1111/micc.12691. Epub 2021 Mar 11. Microcirculation. 2021. PMID: 33655585 Free PMC article.
-
The Foundation for Engineering a Pancreatic Islet Niche.Front Endocrinol (Lausanne). 2022 May 4;13:881525. doi: 10.3389/fendo.2022.881525. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35600597 Free PMC article. Review.
-
Pancreatic Crosstalk in the Disease Setting: Understanding the Impact of Exocrine Disease on Endocrine Function.Compr Physiol. 2024 Mar 29;14(2):5371-5387. doi: 10.1002/cphy.c230008. Compr Physiol. 2024. PMID: 39109973 Free PMC article. Review.
-
Multiscale and multimodal imaging for three-dimensional vascular and histomorphological organ structure analysis of the pancreas.Sci Rep. 2024 May 2;14(1):10136. doi: 10.1038/s41598-024-60254-9. Sci Rep. 2024. PMID: 38698049 Free PMC article.
References
-
- Fujita T. Insulo-acinar portal system in the horse pancreas. Arch Histol Jpn 1973;35:161–171 - PubMed
-
- Murakami T, Fujita T. Microcirculation of the rat pancreas, with special reference to the insulo-acinar portal and insulo-venous drainage systems: a further scanning electron microscope study of corrosion casts. Arch Histol Cytol 1992;55:453–476 - PubMed
-
- Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 1982;31:883–889 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

