Repeated hypoglycemia remodels neural inputs and disrupts mitochondrial function to blunt glucose-inhibited GHRH neuron responsiveness
- PMID: 33148883
- PMCID: PMC7710320
- DOI: 10.1172/jci.insight.133488
Repeated hypoglycemia remodels neural inputs and disrupts mitochondrial function to blunt glucose-inhibited GHRH neuron responsiveness
Abstract
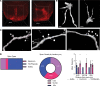
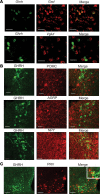
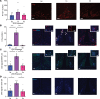
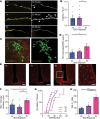
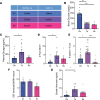
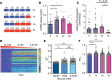
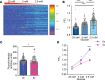
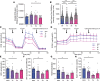
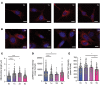
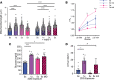
Hypoglycemia is a frequent complication of diabetes, limiting therapy and increasing morbidity and mortality. With recurrent hypoglycemia, the counterregulatory response (CRR) to decreased blood glucose is blunted, resulting in hypoglycemia-associated autonomic failure (HAAF). The mechanisms leading to these blunted effects are only poorly understood. Here, we report, with ISH, IHC, and the tissue-clearing capability of iDISCO+, that growth hormone releasing hormone (GHRH) neurons represent a unique population of arcuate nucleus neurons activated by glucose deprivation in vivo. Repeated glucose deprivation reduces GHRH neuron activation and remodels excitatory and inhibitory inputs to GHRH neurons. We show that low glucose sensing is coupled to GHRH neuron depolarization, decreased ATP production, and mitochondrial fusion. Repeated hypoglycemia attenuates these responses during low glucose. By maintaining mitochondrial length with the small molecule mitochondrial division inhibitor-1, we preserved hypoglycemia sensitivity in vitro and in vivo. Our findings present possible mechanisms for the blunting of the CRR, significantly broaden our understanding of the structure of GHRH neurons, and reveal that mitochondrial dynamics play an important role in HAAF. We conclude that interventions targeting mitochondrial fission in GHRH neurons may offer a new pathway to prevent HAAF in patients with diabetes.
Keywords: Diabetes; Metabolism; Neuroscience.
Conflict of interest statement
Figures
Similar articles
-
Sex-Dimorphic Effects of Hypoglycemia on Metabolic Sensor mRNA Expression in Ventromedial Hypothalamic Nucleus-Dorsomedial Division (VMNdm) Growth Hormone-Releasing Hormone Neurons.ACS Chem Neurosci. 2024 Jun 5;15(11):2350-2358. doi: 10.1021/acschemneuro.4c00206. Epub 2024 May 17. ACS Chem Neurosci. 2024. PMID: 38757688
-
GHRH Neurons from the Ventromedial Hypothalamic Nucleus Provide Dynamic and Sex-Specific Input to the Brain Glucose-Regulatory Network.Neuroscience. 2023 Oct 1;529:73-87. doi: 10.1016/j.neuroscience.2023.08.006. Epub 2023 Aug 10. Neuroscience. 2023. PMID: 37572878 Free PMC article.
-
Ghrelin does not impact the blunted counterregulatory response to recurrent hypoglycemia in mice.Front Endocrinol (Lausanne). 2023 Jun 2;14:1181856. doi: 10.3389/fendo.2023.1181856. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37334290 Free PMC article.
-
Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF).Diabetes Metab Res Rev. 2003 Sep-Oct;19(5):348-56. doi: 10.1002/dmrr.404. Diabetes Metab Res Rev. 2003. PMID: 12951642 Review.
-
Potential approaches to prevent hypoglycemia-associated autonomic failure.J Investig Med. 2018 Mar;66(3):641-647. doi: 10.1136/jim-2017-000582. Epub 2017 Nov 14. J Investig Med. 2018. PMID: 29141871 Free PMC article. Review.
Cited by
-
Acute severe hypoglycemia alters mouse brain microvascular proteome.J Cereb Blood Flow Metab. 2024 Apr;44(4):556-572. doi: 10.1177/0271678X231212961. Epub 2023 Nov 9. J Cereb Blood Flow Metab. 2024. PMID: 37944245 Free PMC article.
-
Recent advances in understanding hypothalamic control of defensive responses to hypoglycaemia.Curr Opin Endocr Metab Res. 2022 Jun;24:100353. doi: 10.1016/j.coemr.2022.100353. Curr Opin Endocr Metab Res. 2022. PMID: 39183767 Free PMC article. Review.
-
Growth hormone-releasing hormone and its analogues in health and disease.Nat Rev Endocrinol. 2025 Mar;21(3):180-195. doi: 10.1038/s41574-024-01052-1. Epub 2024 Nov 13. Nat Rev Endocrinol. 2025. PMID: 39537825 Review.
-
GHRH in diabetes and metabolism.Rev Endocr Metab Disord. 2025 Jun;26(3):413-426. doi: 10.1007/s11154-024-09930-9. Epub 2024 Nov 19. Rev Endocr Metab Disord. 2025. PMID: 39560873 Free PMC article. Review.
-
Characterization and Regulation of the Neonatal Growth Hormone Surge.Endocrinology. 2024 Oct 30;165(12):bqae140. doi: 10.1210/endocr/bqae140. Endocrinology. 2024. PMID: 39446366
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

