Transient enlargement of brain ventricles during relapsing-remitting multiple sclerosis and experimental autoimmune encephalomyelitis
- PMID: 33148886
- PMCID: PMC7710287
- DOI: 10.1172/jci.insight.140040
Transient enlargement of brain ventricles during relapsing-remitting multiple sclerosis and experimental autoimmune encephalomyelitis
Abstract
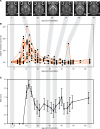
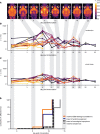
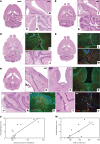
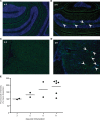
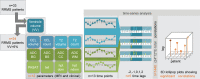
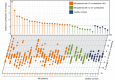
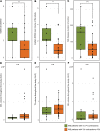
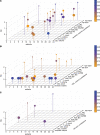
The brain ventricles are part of the fluid compartments bridging the CNS with the periphery. Using MRI, we previously observed a pronounced increase in ventricle volume (VV) in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). Here, we examined VV changes in EAE and MS patients in longitudinal studies with frequent serial MRI scans. EAE mice underwent serial MRI for up to 2 months, with gadolinium contrast as a proxy of inflammation, confirmed by histopathology. We performed a time-series analysis of clinical and MRI data from a prior clinical trial in which RRMS patients underwent monthly MRI scans over 1 year. VV increased dramatically during preonset EAE, resolving upon clinical remission. VV changes coincided with blood-brain barrier disruption and inflammation. VV was normal at the termination of the experiment, when mice were still symptomatic. The majority of relapsing-remitting MS (RRMS) patients showed dynamic VV fluctuations. Patients with contracting VV had lower disease severity and a shorter duration. These changes demonstrate that VV does not necessarily expand irreversibly in MS but, over short time scales, can expand and contract. Frequent monitoring of VV in patients will be essential to disentangle the disease-related processes driving short-term VV oscillations from persistent expansion resulting from atrophy.
Keywords: Autoimmunity; Inflammation; Mouse models; Multiple sclerosis; Neuroimaging.
Conflict of interest statement
Figures

Similar articles
-
Perivenular brain lesions in a primate multiple sclerosis model at 7-tesla magnetic resonance imaging.Mult Scler. 2014 Jan;20(1):64-71. doi: 10.1177/1352458513492244. Epub 2013 Jun 17. Mult Scler. 2014. PMID: 23773983 Free PMC article.
-
Distinct pathological patterns in relapsing-remitting and chronic models of experimental autoimmune enchephalomyelitis and the neuroprotective effect of glatiramer acetate.J Autoimmun. 2011 Nov;37(3):228-41. doi: 10.1016/j.jaut.2011.06.003. Epub 2011 Jul 14. J Autoimmun. 2011. PMID: 21752599
-
miR-142-3p Is a Key Regulator of IL-1β-Dependent Synaptopathy in Neuroinflammation.J Neurosci. 2017 Jan 18;37(3):546-561. doi: 10.1523/JNEUROSCI.0851-16.2016. J Neurosci. 2017. PMID: 28100738 Free PMC article.
-
Experimental models of relapsing-remitting multiple sclerosis: current concepts and perspective.Curr Neurovasc Res. 2005 Oct;2(4):349-62. doi: 10.2174/156720205774322601. Curr Neurovasc Res. 2005. PMID: 16181126 Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
Contribution of preclinical MRI to responsible animal research: living up to the 3R principle.MAGMA. 2021 Aug;34(4):469-474. doi: 10.1007/s10334-021-00929-w. Epub 2021 May 19. MAGMA. 2021. PMID: 34009521 Free PMC article. No abstract available.
-
Longitudinal evaluation of structural brain alterations in two established mouse models of Gulf War Illness.Front Neurosci. 2024 Sep 6;18:1465701. doi: 10.3389/fnins.2024.1465701. eCollection 2024. Front Neurosci. 2024. PMID: 39308947 Free PMC article.
-
Impairment of spinal CSF flow precedes immune cell infiltration in an active EAE model.J Neuroinflammation. 2024 Oct 23;21(1):272. doi: 10.1186/s12974-024-03247-9. J Neuroinflammation. 2024. PMID: 39444001 Free PMC article.
-
A review on multiple sclerosis prognostic findings from imaging, inflammation, and mental health studies.Front Hum Neurosci. 2023 May 11;17:1151531. doi: 10.3389/fnhum.2023.1151531. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37250694 Free PMC article. Review.
-
Choroid plexus volume in multiple sclerosis: a systematic review and meta-analysis of an emerging imaging biomarker.Eur J Med Res. 2025 Aug 18;30(1):762. doi: 10.1186/s40001-025-03026-z. Eur J Med Res. 2025. PMID: 40826366 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

