Elevated CCL2 causes Leydig cell malfunction in metabolic syndrome
- PMID: 33148888
- PMCID: PMC7710294
- DOI: 10.1172/jci.insight.134882
Elevated CCL2 causes Leydig cell malfunction in metabolic syndrome
Abstract
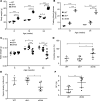
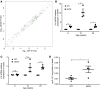
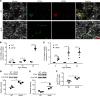
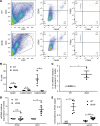
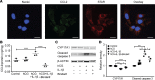
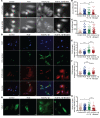
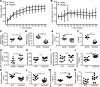
Metabolic syndrome (MetS), which is associated with chronic inflammation, predisposes males to hypogonadism and subfertility. The underlying mechanism of these pathologies remains poorly understood. Homozygous leptin-resistant obese db/db mice are characterized by small testes, low testicular testosterone, and a reduced number of Leydig cells. Here we report that IL-1β, CCL2 (also known as MCP-1), and corticosterone concentrations were increased in the testes of db/db mice relative to those in WT controls. Cultured murine and human Leydig cells responded to cytokine stress with increased CCL2 release and apoptotic signals. Chemical inhibition of CCL2 rescued Leydig cell function in vitro and in db/db mice. Consistently, we found that Ccl2-deficient mice fed with a high-energy diet were protected from testicular dysfunction compared with similarly fed WT mice. Finally, a cohort of infertile men with a history of MetS showed that reduction of CCL2 plasma levels could be achieved by weight loss and was clearly associated with recovery from hypogonadism. Taken together, we conclude that CCL2-mediated chronic inflammation is, to a large extent, responsible for the subfertility in MetS by causing damage to Leydig cells.
Keywords: Chemokines; Endocrinology; Fertility; Reproductive Biology; Urology.
Conflict of interest statement
Figures


Similar articles
-
Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice.Int J Mol Sci. 2016 Jan 13;17(1):96. doi: 10.3390/ijms17010096. Int J Mol Sci. 2016. PMID: 26771609 Free PMC article.
-
Leydig cell dysfunction, systemic inflammation and metabolic syndrome in long-term testicular cancer survivors.Eur J Cancer. 2017 Oct;84:9-17. doi: 10.1016/j.ejca.2017.07.006. Epub 2017 Jul 31. Eur J Cancer. 2017. PMID: 28772110
-
Estrogen promotes Leydig cell engulfment by macrophages in male infertility.J Clin Invest. 2014 Jun;124(6):2709-21. doi: 10.1172/JCI59901. Epub 2014 Apr 24. J Clin Invest. 2014. PMID: 24762434 Free PMC article.
-
Implications of leptin in neuroendocrine regulation of male reproduction.Reprod Biol. 2013 Mar;13(1):1-14. doi: 10.1016/j.repbio.2012.12.001. Epub 2013 Jan 16. Reprod Biol. 2013. PMID: 23522066 Review.
-
Metabolic and cardiovascular risk factors in Klinefelter syndrome.Am J Med Genet C Semin Med Genet. 2020 Jun;184(2):334-343. doi: 10.1002/ajmg.c.31792. Epub 2020 May 26. Am J Med Genet C Semin Med Genet. 2020. PMID: 32452627 Review.
Cited by
-
The Role of Testosterone in the Elderly: What Do We Know?Int J Mol Sci. 2022 Mar 24;23(7):3535. doi: 10.3390/ijms23073535. Int J Mol Sci. 2022. PMID: 35408895 Free PMC article. Review.
-
Utility of CD138/syndecan-1 immunohistochemistry for localization of plasmacytes is tissue-dependent in B6 mice.BMC Res Notes. 2022 Jun 25;15(1):219. doi: 10.1186/s13104-022-06100-5. BMC Res Notes. 2022. PMID: 35752869 Free PMC article.
-
Region-specific roles of stress-activated protein kinase MKK7 in the developing and maturing murine brain.Sci Rep. 2025 Jul 1;15(1):20504. doi: 10.1038/s41598-025-05388-0. Sci Rep. 2025. PMID: 40594443 Free PMC article.
-
High-throughput proteomic characterization of seminal plasma from bulls with contrasting semen quality.3 Biotech. 2023 Feb;13(2):60. doi: 10.1007/s13205-023-03474-6. Epub 2023 Jan 26. 3 Biotech. 2023. PMID: 36714547 Free PMC article.
-
Astragalin alleviates oligoasthenospermia via promoting nuclear translocation of Nrf2 and reducing ferroptosis of testis.Heliyon. 2024 Oct 3;10(19):e38778. doi: 10.1016/j.heliyon.2024.e38778. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39444397 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

