Preconditioning the immature lung with enhanced Nrf2 activity protects against oxidant-induced hypoalveolarization in mice
- PMID: 33149211
- PMCID: PMC7642393
- DOI: 10.1038/s41598-020-75834-8
Preconditioning the immature lung with enhanced Nrf2 activity protects against oxidant-induced hypoalveolarization in mice
Abstract
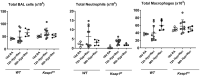
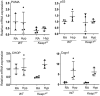
Bronchopulmonary dysplasia (BPD) is a chronic disease of preterm babies with poor clinical outcomes. Nrf2 transcription factor is crucial for cytoprotective response, whereas Keap1-an endogenous inhibitor of Nrf2 signaling-dampens these protective responses. Nrf2-sufficient (wild type) newborn mice exposed to hyperoxia develop hypoalveolarization, which phenocopies human BPD, and Nrf2 deficiency worsens it. In this study, we used PND1 pups bearing bearing hypomorphic Keap1 floxed alleles (Keap1f/f) with increased levels of Nrf2 to test the hypothesis that constitutive levels of Nrf2 in the premature lung are insufficient to mitigate hyperoxia-induced hypoalveolarization. Both wildtype and Keap1f/f pups at PND1 were exposed to hyperoxia for 72 h and then allowed to recover at room air for two weeks (at PND18), sacrificed, and lung hypoalveolarization and inflammation assessed. Hyperoxia-induced lung hypoalveolarization was remarkably lower in Keap1f/f pups than in wildtype counterparts (28.9% vs 2.4%, wildtype vs Keap1f/f). Likewise, Keap1f/f pups were protected against prolonged (96 h) hyperoxia-induced hypoalveolarization. However, there were no differences in hyperoxia-induced lung inflammatory response immediately after exposure or at PND18. Lack of hypoalveolarization in Keap1f/f pups was accompanied by increased levels of expression of antioxidant genes and GSH as assessed immediately following hyperoxia. Keap1 knockdown resulted in upregulation of lung cell proliferation postnatally but had opposing effects following hyperoxia. Collectively, our study demonstrates that augmenting endogenous Nrf2 activation by targeting Keap1 may provide a physiological way to prevent hypoalveolarization associated with prematurity.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Myeloid Nrf2 Protects against Neonatal Oxidant-Stress-Induced Lung Inflammation and Alveolar Simplification in Mice.Antioxidants (Basel). 2024 Jun 7;13(6):698. doi: 10.3390/antiox13060698. Antioxidants (Basel). 2024. PMID: 38929137 Free PMC article.
-
Mechanism of oxidative stress and Keap-1/Nrf2 signaling pathway in bronchopulmonary dysplasia.Medicine (Baltimore). 2020 Jun 26;99(26):e20433. doi: 10.1097/MD.0000000000020433. Medicine (Baltimore). 2020. PMID: 32590729 Free PMC article.
-
Effects of hyperoxia exposure on the expression of Nrf2 and heme oxygenase-1 in lung tissues of premature rats.Mol Cell Probes. 2020 Jun;51:101529. doi: 10.1016/j.mcp.2020.101529. Epub 2020 Feb 6. Mol Cell Probes. 2020. PMID: 32036037
-
The Role of Sphingolipid Signaling in Oxidative Lung Injury and Pathogenesis of Bronchopulmonary Dysplasia.Int J Mol Sci. 2022 Jan 23;23(3):1254. doi: 10.3390/ijms23031254. Int J Mol Sci. 2022. PMID: 35163176 Free PMC article. Review.
-
Nrf2 protects against airway disorders.Toxicol Appl Pharmacol. 2010 Apr 1;244(1):43-56. doi: 10.1016/j.taap.2009.07.024. Epub 2009 Jul 29. Toxicol Appl Pharmacol. 2010. PMID: 19646463 Review.
Cited by
-
Regulating NLRP3 Inflammasome-Induced Pyroptosis via Nrf2: TBHQ Limits Hyperoxia-Induced Lung Injury in a Mouse Model of Bronchopulmonary Dysplasia.Inflammation. 2023 Dec;46(6):2386-2401. doi: 10.1007/s10753-023-01885-4. Epub 2023 Aug 9. Inflammation. 2023. PMID: 37556072 Free PMC article.
-
Selenium Deficiency Exacerbates Hyperoxia-Induced Lung Injury in Newborn C3H/HeN Mice.Antioxidants (Basel). 2024 Mar 25;13(4):391. doi: 10.3390/antiox13040391. Antioxidants (Basel). 2024. PMID: 38671839 Free PMC article.
-
Oxygen Toxicity to the Immature Lung-Part I: Pathomechanistic Understanding and Preclinical Perspectives.Int J Mol Sci. 2021 Oct 12;22(20):11006. doi: 10.3390/ijms222011006. Int J Mol Sci. 2021. PMID: 34681665 Free PMC article. Review.
-
Effects of Antioxidants in Human Milk on Bronchopulmonary Dysplasia Prevention and Treatment: A Review.Front Nutr. 2022 Jul 18;9:924036. doi: 10.3389/fnut.2022.924036. eCollection 2022. Front Nutr. 2022. PMID: 35923207 Free PMC article. Review.
-
Molecular mechanisms of cell death in bronchopulmonary dysplasia.Apoptosis. 2023 Feb;28(1-2):39-54. doi: 10.1007/s10495-022-01791-4. Epub 2022 Nov 11. Apoptosis. 2023. PMID: 36369365 Review.
References
-
- Fanaroff AA, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007;196(147):e141–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

