Distinct effects of different matrix proteoglycans on collagen fibrillogenesis and cell-mediated collagen reorganization
- PMID: 33149218
- PMCID: PMC7642422
- DOI: 10.1038/s41598-020-76107-0
Distinct effects of different matrix proteoglycans on collagen fibrillogenesis and cell-mediated collagen reorganization
Abstract
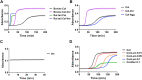
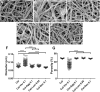
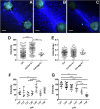
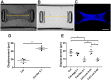
The extracellular matrix (ECM) is a complex mixture composed of fibrillar collagens as well as additional protein and carbohydrate components. Proteoglycans (PGs) contribute to the heterogeneity of the ECM and play an important role in its structure and function. While the small leucine rich proteoglycans (SLRPs), including decorin and lumican, have been studied extensively as mediators of collagen fibrillogenesis and organization, the function of large matrix PGs in collagen matrices is less well known. In this study, we showed that different matrix PGs have distinct roles in regulating collagen behaviors. We found that versican, a large chondroitin sulfate PG, promotes collagen fibrillogenesis in a turbidity assay and upregulates cell-mediated collagen compaction and reorganization, whereas aggrecan, a structurally-similar large PG, has different and often opposing effects on collagen. Compared to versican, decorin and lumican also have distinct functions in regulating collagen behaviors. The different ways in which matrix PGs interact with collagen have important implications for understanding the role of the ECM in diseases such as fibrosis and cancer, and suggest that matrix PGs are potential therapeutic targets.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

