Inhibition of red blood cell development by arsenic-induced disruption of GATA-1
- PMID: 33149232
- PMCID: PMC7643154
- DOI: 10.1038/s41598-020-76118-x
Inhibition of red blood cell development by arsenic-induced disruption of GATA-1
Abstract
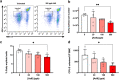
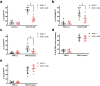
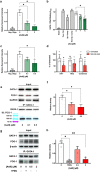
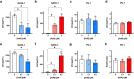
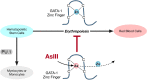
Anemia is a hematological disorder that adversely affects the health of millions of people worldwide. Although many variables influence the development and exacerbation of anemia, one major contributing factor is the impairment of erythropoiesis. Normal erythropoiesis is highly regulated by the zinc finger transcription factor GATA-1. Disruption of the zinc finger motifs in GATA-1, such as produced by germline mutations, compromises the function of this critical transcription factor and causes dyserythropoietic anemia. Herein, we utilize a combination of in vitro and in vivo studies to provide evidence that arsenic, a widespread environmental toxicant, inhibits erythropoiesis likely through replacing zinc within the zinc fingers of the critical transcription factor GATA-1. We found that arsenic interacts with the N- and C-terminal zinc finger motifs of GATA-1, causing zinc loss and inhibition of DNA and protein binding activities, leading to dyserythropoiesis and an imbalance of hematopoietic differentiation. For the first time, we show that exposures to a prevalent environmental contaminant compromises the function of a key regulatory factor in erythropoiesis, producing effects functionally similar to inherited GATA-1 mutations. These findings highlight a novel molecular mechanism by which arsenic exposure may cause anemia and provide critical insights into potential prevention and intervention for arsenic-related anemias.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Organization, World Health. The Global Prevalence of Anaemia in 2011.www.who.int/nutrition/publications/micronutrients/global_prevalence_anae... (2015). Accessed 17 Dec 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

