Lung Cancer Combination Treatment: Evaluation of the Synergistic Effect of Cisplatin Prodrug, Vinorelbine and Retinoic Acid When Co-Encapsulated in a Multi-Layered Nano-Platform
- PMID: 33149550
- PMCID: PMC7602907
- DOI: 10.2147/DDDT.S251749
Lung Cancer Combination Treatment: Evaluation of the Synergistic Effect of Cisplatin Prodrug, Vinorelbine and Retinoic Acid When Co-Encapsulated in a Multi-Layered Nano-Platform
Abstract
Purpose: Lung cancer remains the leading cancer-associated deaths worldwide. Cisplatin (CIS) was often used in combination with other drugs for the treatment of non-small cell lung cancer (NSCLC). Prodrug is an effective strategy to improve the efficiency of drugs and reduce the toxicity. The aim of this study was to prepare and characterize CIS prodrug, vinorelbine (VNR), and all-trans retinoic acid (ATRA) co-delivered multi-layered nano-platform, evaluating their antitumor activity in vitro and in vivo.
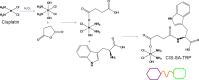
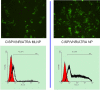
Methods: Cisplatin prodrug (CISP) was synthesized. A multi-layered nano-platform contained CISP, VNR and ATRA were prepared and named CISP/VNR/ATRA MLNP. The physicochemical properties of CISP/VNR/ATRA MLNP were investigated. In vitro cytotoxicity against CIS-resistant NSCLC cells (A549/CIS cells) and Human normal lung epithelial cells (BEAS-2B cells) was investigated, and in vivo anti-tumor efficiency was evaluated on mice bearing A549/CIS cells xenografts.
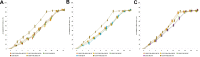
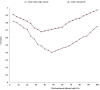
Results: CISP/VNR/ATRA MLNP were spherical particles with particle size and zeta potential of 158 nm and 12.3 mV. CISP/VNR/ATRA MLNP (81.36%) was uptake by cancer cells in vitro. CISP/VNR/ATRA MLNP could significantly inhibit the in vivo antitumor growth and suspended the tumor volume from 1440 mm3 to 220 mm3.
Conclusion: It could be concluded that the CISP/VNR/ATRA MLNP may be used as a promising system for lung cancer combination treatment.
Keywords: cisplatin prodrug; combination treatment; lung cancer; multi-layered nano-platform; synergistic effect.
© 2020 Liang et al.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures







Similar articles
-
Synergistic Combination Chemotherapy of Lung Cancer: Cisplatin and Doxorubicin Conjugated Prodrug Loaded, Glutathione and pH Sensitive Nanocarriers.Drug Des Devel Ther. 2020 Nov 25;14:5205-5215. doi: 10.2147/DDDT.S260253. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33268983 Free PMC article.
-
Combination Chemotherapy of Lung Cancer - Co-Delivery of Docetaxel Prodrug and Cisplatin Using Aptamer-Decorated Lipid-Polymer Hybrid Nanoparticles.Drug Des Devel Ther. 2020 Jun 9;14:2249-2261. doi: 10.2147/DDDT.S246574. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32606595 Free PMC article.
-
Combination Therapy of Lung Cancer Using Layer-by-Layer Cisplatin Prodrug and Curcumin Co-Encapsulated Nanomedicine.Drug Des Devel Ther. 2020 Jun 9;14:2263-2274. doi: 10.2147/DDDT.S241291. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32606596 Free PMC article.
-
[Properties of antitumor activity of vinorelbine tartrate, a new vinca alkaloid antitumor agent].Nihon Yakurigaku Zasshi. 2000 Oct;116(4):215-23. doi: 10.1254/fpj.116.215. Nihon Yakurigaku Zasshi. 2000. PMID: 11084918 Review. Japanese.
-
Nano-mediated strategy for targeting and treatment of non-small cell lung cancer (NSCLC).Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2769-2792. doi: 10.1007/s00210-023-02522-5. Epub 2023 May 23. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37219615 Review.
Cited by
-
Prostate Cancer Therapy Using Docetaxel and Formononetin Combination: Hyaluronic Acid and Epidermal Growth Factor Receptor Targeted Peptide Dual Ligands Modified Binary Nanoparticles to Facilitate the in vivo Anti-Tumor Activity.Drug Des Devel Ther. 2022 Aug 11;16:2683-2693. doi: 10.2147/DDDT.S366622. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35983428 Free PMC article.
-
Combination Therapy of Metastatic Castration-Recurrent Prostate Cancer: Hyaluronic Acid Decorated, Cabazitaxel-Prodrug and Orlistat Co-Loaded Nano-System.Drug Des Devel Ther. 2021 Aug 20;15:3605-3616. doi: 10.2147/DDDT.S306684. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34447241 Free PMC article.
-
Nanocarrier-mediated cancer therapy with cisplatin: A meta-analysis with a promising new paradigm.Heliyon. 2024 Mar 27;10(7):e28171. doi: 10.1016/j.heliyon.2024.e28171. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 39839154 Free PMC article.
-
Dual-Drug Nanosystem: Etoposide Prodrug and Cisplatin Coloaded Nanostructured Lipid Carriers for Lung Cancer Therapy.Drug Des Devel Ther. 2022 Dec 5;16:4139-4149. doi: 10.2147/DDDT.S386100. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36506793 Free PMC article.
References
-
- GLOBOCAN. 2018. Available from: http://gco.iarc.fr/. accessed on 12April 2019.
-
- Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/., November 2017. SEER data submission, posted to the SEER web site, April 2018.
-
- Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: american College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5Suppl):e278S–e313S. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

