Development of a Severity Classification System for Sickle Cell Disease
- PMID: 33149635
- PMCID: PMC7604906
- DOI: 10.2147/CEOR.S276121
Development of a Severity Classification System for Sickle Cell Disease
Abstract
Purpose: There is no well-accepted classification system of overall sickle cell disease (SCD) severity. We sought to develop a system that could be tested as a clinical outcome predictor.
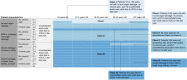
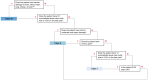
Patients and methods: Using validated methodology (RAND/UCLA modified Delphi panel), 10 multi-disciplinary expert clinicians collaboratively developed 180 simplified patient histories and rated each on multiple axes (estimated clinician follow-up frequency, risk of complications or death, quality of life, overall disease severity). Using ratings on overall disease severity, we developed a 3-level severity classification system ranging from Class I (least severe) to Class III (most severe).
Results: The system defines patients as Class I who are 8-40 years with no end organ damage, no chronic pain, and ≤4 unscheduled acute care visits due to vaso-occlusive crises (VOC) in the last year. Patients <8 or >40 years with no end organ damage, no chronic pain, and <2 unscheduled acute care visits are also considered Class I. Patients any age with ≥5 unscheduled acute care visits and/or with severe damage to bone, retina, heart, lung, kidney, or brain are classified as Class III (except patients ≥25 years with severe retinopathy, no chronic pain, and 0-1 unscheduled acute care visits, who are considered Class II). Patients not meeting these Class I or III definitions are classified as Class II.
Conclusion: This system consolidates patient characteristics into homogenous groups with respect to disease state to support clinical decision-making. The system is consistent with existing literature that increased unscheduled acute care visits and organ damage translate into clinically significant patient morbidity. Studies to further validate this system are planned.
Keywords: chronic pain; disease severity; expert panel; organ damage; vaso-occlusive crises.
© 2020 Shah et al.
Conflict of interest statement
Nirmish Shah has received funding from Novartis (honoraria, consultancy, research funding, speaker’s bureau), Alexion (speaker’s bureau), Global Blood Therapeutics (research funding), CSL Behring and Bluebird Bio (consultant). David Beenhouwer, Michael Broder, Sarah N Gibbs, and Irina Yermilov are employees of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by Novartis to conduct the research described in this manuscript. David Beenhouwer, Michael Broder, Sarah N Gibbs, and Irina Yermilov are employees of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by AbbVie, Akcea, ASPC, Amgen, AstraZeneca, BMS, Boston Scientific Corporation, Celgene, Eisai, Ethicon, GRAIL, Helsinn, Illumina, Innovation and Value Initiative, Ionis, Jazz, Kite, Novartis, Otsuka, Pathnostics, PhRMA, Prothena, Sage, Verde Technologies, Genentech, Greenwich Biosciences, Mirum Pharmaceuticals, Sanofi US Services, Sunovion Pharmaceuticals, and Dompe US to conduct research outside of the submitted work. Lanetta Bronte-Hall has received funding from Novartis (honoraria, consulting), Global Blood Therapeutics (research funding), Pfizer (consultancy and research support), and BlueBird Bio (research funding). Laura M De Castro has received funding from Novartis (honoraria, membership on Board of Directors or advisory committees), Global Blood Therapeutics (membership on Board of Directors or advisory committees), and Pfizer (consultancy). She has received research support from Global Blood Therapeutics, Novartis, Pfizer, and Bayer. Victor Gordeuk has received funding from Global Blood Therapeutics (honoraria, consultancy, research funding), Emmaus (honoraria, consultancy), Novartis (honoraria, consultancy, research funding), Modus Therapeutics (honoraria, consultancy), Pfizer (research funding), Inctye (research funding), CSL Behring (honoraria, consultancy, research funding), Ironwood (research funding), and Imara (research funding). Julie Kanter has received funding from Novartis (honoraria, membership on advisory committees). She has also received funding from AstraZeneca (steering committee), Imara (honoraria), Modus Therapeutics (honoraria), and Global Blood Therapeutics (travel). Elizabeth S Klings has received funding from Novartis (honoraria). She has received research support from Actelion, Reata, Incyte, Bayer, Arena/United Therapeutics. She has received funding from Pfizer (membership on Acute Chest Syndrome adjudication committee for Rivipansel clinical trial) and Micelle (Data and Safety Monitoring Board). Thokozeni Lipato has received funding from Novartis (honoraria) and Global Blood Therapeutics (speaker’s bureau). Deepa Manwani has received funding from Novartis (honoraria, consultancy), Pfizer (consultancy), and Global Blood Therapeutics (consultancy, research funding). Brigid Scullin has received funding from Novartis (honoraria, consultancy). Wally R Smith has received funding from Novartis (honoraria, consultancy) and reports funding as an investigator for NHLBI, HRSA, PCORI, Pfizer, Novartis, Emmaus, Imara, and Shire; consultant for Novartis, Pfizer, Global Blood Therapeutics, and Emmaus. The authors report no other conflicts of interest in this work. Selected components of this work were presented at the 2019 American Society of Hematology (ASH) Annual Meeting in Orlando, Florida, on December 8, 2019.
Figures

References
-
- Day SW. Development and evaluation of a sickle cell disease assessment instrument. Pediatr Nurs. 2004;30(6). - PubMed
LinkOut - more resources
Full Text Sources

