HMGB-1/RAGE signaling inhibition by dioscin attenuates hippocampal neuron damage induced by oxygen-glucose deprivation/reperfusion
- PMID: 33149785
- PMCID: PMC7604738
- DOI: 10.3892/etm.2020.9361
HMGB-1/RAGE signaling inhibition by dioscin attenuates hippocampal neuron damage induced by oxygen-glucose deprivation/reperfusion
Abstract
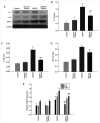
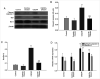
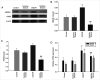
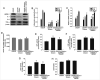
Cerebral ischemia is one of the most common clinical diseases characterized by high morbidity and mortality. Neurocyte apoptosis and a cascade of inflammatory signals following cerebral ischemia-reperfusion injury (IRI) may contribute to secondary brain damage, resulting in severe neurological damage. It has been reported that dioscin, a natural steroid saponin, exerts anti-inflammatory properties against different diseases. The present study aimed to investigate the role of dioscin in oxygen-glucose deprivation/reperfusion (OGD/R) induction in hippocampal cells in vitro and in vivo. For the in vitro study, hippocampal cells were collected from rat embryos of gestational age of E18. The oxygen-glucose deprivation model in primary hippocampal neurons was used to mimic cerebral IRI in vitro. To select the optimum dioscin concentration and acting time, cell viability was evaluated by a Cell Counting Kit-8 (CCK-8) assay. Neurons subjected to OGD/R were treated with dioscin and the inflammatory cytokines, high mobility group box chromosomal protein 1 (HMGB-1)/receptor for advanced glycation end products (RAGE) signaling molecules and apoptosis-associated genes were determined. The intracellular reactive oxygen species (ROS) generation was detected. Furthermore, the effects of dioscin on the antioxidant defense mechanisms were evaluated by measuring the activity of glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT) and the glutathione (GSH)/glutathione disulphide (GSSG) ratio. In addition, OGD/R-induced cells were transfected with pcDNA3.1-HMGB-1 and treated with dioscin, and the neuronal cell apoptosis rate was determined using a terminal deoxynucleotidyl transferase-mediated 2-deoxyuridine 5-triphosphate-biotin nick-end labeling (TUNEL) assay. The mRNA and protein expression levels of the inflammatory factors were measured using real-time quantitative polymerase chain reaction (RT-qPCR) and western blot analysis, respectively. For the in vivo investigation, the oxidation and anti-oxidation system in rat hippocampal tissue was evaluated by detecting the expression of the aforementioned oxidative stress-associated proteins, 3-NT as well as 8-oxo-deoxyguanosine (8-OHdG). In the hippocampal region, the apoptotic rate was determined using a TUNEL assay. The results demonstrated that dioscin at a dose of 400 ng/ml significantly reversed the increase in the expression levels of the inflammatory factors and attenuated those of apoptotic cytokines induced by OGD/R. Additionally, dioscin notably reversed the OGD/R-mediated activation of the HMGB-1/RAGE signaling pathway in vitro and in vivo. Cell treatment with dioscin significantly attenuated ROS production and increased the activity of antioxidant enzymes. Additionally, increasing the expression of HMGB-1 inhibited the protective effects of dioscin on cell apoptosis in the OGD/R-induced neurons. Furthermore, HMGB-1 overexpression reversed the antiapoptotic and anti-inflammatory effects of dioscin on neurons. The results of the present study indicated that dioscin exerted anti-inflammatory, antiapoptotic and antioxidant effects via the HMGB-1/RAGE signaling pathway. These results suggest a novel perspective of the protective effects of dioscin as a prospective remedial factor for IRI.
Keywords: apoptosis; dioscin; high mobility group box-1 protein; inflammation; oxygen-glucose deprivation.
Copyright: © Liu et al.
Figures



Similar articles
-
Protective effect of mesenchymal stem cell-derived exosomal treatment of hippocampal neurons against oxygen-glucose deprivation/reperfusion-induced injury.World J Emerg Med. 2022;13(1):46-53. doi: 10.5847/wjem.j.1920-8642.2022.015. World J Emerg Med. 2022. PMID: 35003415 Free PMC article.
-
Dioscin ameliorates cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via HMGB-1 inhibition.Free Radic Biol Med. 2015 Jul;84:103-115. doi: 10.1016/j.freeradbiomed.2015.03.003. Epub 2015 Mar 13. Free Radic Biol Med. 2015. PMID: 25772012
-
Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway.Chem Biol Interact. 2018 Mar 25;284:32-40. doi: 10.1016/j.cbi.2018.02.017. Epub 2018 Feb 16. Chem Biol Interact. 2018. PMID: 29454613
-
Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage.Acta Pharm Sin B. 2015 Jan;5(1):8-24. doi: 10.1016/j.apsb.2014.11.002. Epub 2015 Jan 24. Acta Pharm Sin B. 2015. PMID: 26579420 Free PMC article. Review.
-
Construction of PLGA/JNK3-shRNA nanoparticles and their protective role in hippocampal neuron apoptosis induced by oxygen and glucose deprivation.RSC Adv. 2018 Jun 1;8(36):20108-20116. doi: 10.1039/c8ra00679b. eCollection 2018 May 30. RSC Adv. 2018. PMID: 35541669 Free PMC article. Review.
Cited by
-
Biliverdin Protects Against Cerebral Ischemia/Reperfusion Injury by Regulating the miR-27a-3p/Rgs1 Axis.Neuropsychiatr Dis Treat. 2021 Apr 22;17:1165-1181. doi: 10.2147/NDT.S300773. eCollection 2021. Neuropsychiatr Dis Treat. 2021. PMID: 33911865 Free PMC article.
-
Soluble receptor for advanced glycation end products protects from ischemia- and reperfusion-induced acute kidney injury.Biol Open. 2022 Jan 15;11(1):bio058852. doi: 10.1242/bio.058852. Epub 2022 Feb 4. Biol Open. 2022. PMID: 34812852 Free PMC article.
-
Melissa officinalis Regulates Lipopolysaccharide-Induced BV2 Microglial Activation via MAPK and Nrf2 Signaling.J Microbiol Biotechnol. 2024 Dec 28;34(12):2474-2483. doi: 10.4014/jmb.2409.09020. Epub 2024 Oct 29. J Microbiol Biotechnol. 2024. PMID: 39467696 Free PMC article.
-
Maltol inhibits oxygen glucose deprivation‑induced chromatinolysis in SH‑SY5Y cells by maintaining pyruvate level.Mol Med Rep. 2023 Mar;27(3):75. doi: 10.3892/mmr.2023.12962. Epub 2023 Feb 17. Mol Med Rep. 2023. PMID: 36799163 Free PMC article.
-
Adiponectin protects skeletal muscle from ischaemia-reperfusion injury in mice through miR-21/PI3K/Akt signalling pathway.Int Wound J. 2023 May;20(5):1647-1661. doi: 10.1111/iwj.14022. Epub 2022 Nov 25. Int Wound J. 2023. Retraction in: Int Wound J. 2025 Apr;22(4):e70562. doi: 10.1111/iwj.70562. PMID: 36426910 Free PMC article. Retracted.
References
-
- Feigin V, Roth G, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. - DOI - PubMed
-
- Candelario-Jalil E. Injury and repair mechanisms in ischemic stroke: Considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs. 2009;10:644–654. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
