MUTYH Deficiency Is Associated with Attenuated Pulmonary Fibrosis in a Bleomycin-Induced Model
- PMID: 33149810
- PMCID: PMC7603627
- DOI: 10.1155/2020/4828256
MUTYH Deficiency Is Associated with Attenuated Pulmonary Fibrosis in a Bleomycin-Induced Model
Abstract
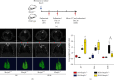
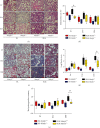
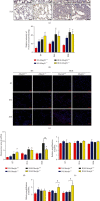
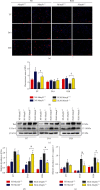
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible lung disease of unknown etiology with limited survival. IPF incidence and prevalence increase significantly with aging, which is associated with an age-related accumulation of oxidative DNA damage. The Mutyh gene is involved in the base excision repair (BER) system, which is critical for repairing the misincorporated adenine that is opposite to the oxidized guanine base, 8-oxoguanine, and maintaining the fidelity of DNA replication. We used Mutyh knockout mice and a bleomycin-induced pulmonary fibrosis model to test the effect of MUTYH deficiency on lesion progression. Unexpectedly, a much less severe lesion of pulmonary fibrosis was observed in Mutyh -/- than in Mutyh +/+ mice, which was supported by assay on protein levels of TGF-β1 and both fibrotic markers, α-SMA and Vimentin, in pulmonary tissues of the model animals. Mechanically, MUTYH deficiency prevented the genomic DNA of pulmonary tissue cells from the buildup of single-strand breaks (SSBs) of DNA and maintained the integrity of mtDNA. Furthermore, increased mitochondrial dynamic regulation and mitophagy were detected in pulmonary tissues of the bleomycin-induced Mutyh -/- model mice, which could reduce the pulmonary epithelial cell apoptosis. Our results suggested that MUTYH deficiency could even induce protective responses of pulmonary tissue under severe oxidative stress.
Copyright © 2020 Qingmin Sun et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Regulation of MUTYH, a DNA Repair Enzyme, in Renal Proximal Tubular Epithelial Cells.Oxid Med Cell Longev. 2015;2015:682861. doi: 10.1155/2015/682861. Epub 2015 Oct 20. Oxid Med Cell Longev. 2015. PMID: 26576226 Free PMC article.
-
Mitochondrial 8-oxoguanine DNA glycosylase mitigates alveolar epithelial cell PINK1 deficiency, mitochondrial DNA damage, apoptosis, and lung fibrosis.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L1084-L1096. doi: 10.1152/ajplung.00069.2019. Epub 2020 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32209025 Free PMC article.
-
Oxidative stress induces different tissue dependent effects on Mutyh-deficient mice.Free Radic Biol Med. 2019 Nov 1;143:482-493. doi: 10.1016/j.freeradbiomed.2019.09.005. Epub 2019 Sep 7. Free Radic Biol Med. 2019. PMID: 31505270
-
The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis.Int J Mol Sci. 2015 Sep 7;16(9):21486-519. doi: 10.3390/ijms160921486. Int J Mol Sci. 2015. PMID: 26370974 Free PMC article. Review.
-
The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis.Exp Lung Res. 2015 Mar;41(2):57-73. doi: 10.3109/01902148.2014.979516. Epub 2014 Dec 16. Exp Lung Res. 2015. PMID: 25514507 Review.
Cited by
-
The Role of DNA Damage and Repair in Idiopathic Pulmonary Fibrosis.Antioxidants (Basel). 2022 Nov 19;11(11):2292. doi: 10.3390/antiox11112292. Antioxidants (Basel). 2022. PMID: 36421478 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

