Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death
- PMID: 33149811
- PMCID: PMC7603626
- DOI: 10.1155/2020/6569728
Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death
Abstract
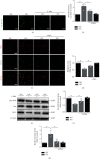
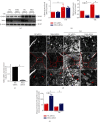
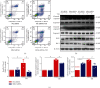
Mitochondrial oxidative stress and dysfunction play an important role of atrial remodeling and atrial fibrillation (AF) in diabetes mellitus. Endoplasmic reticulum (ER) stress has been linked to both physiological and pathological states including diabetes. The aim of this project is to explore the roles of ER stress in hyperglycemia-induced mitochondrial dysfunction and cell death of atrial cardiomyocytes. High glucose upregulated ER stress, mitochondrial oxidative stress, and mitochondria-associated ER membrane (MAM)- enriched proteins (such as glucose-regulated protein 75 (GRP75) and mitofusin-2 (Mfn2)) of primary cardiomyocytes in vitro. Sodium phenylbutyrate (4-PBA) prevented the above changes. Silencing of Mfn2 in HL-1 cells decreased the Ca2+ transfer from ER to mitochondria under ER stress conditions, which were induced by the ER stress agonist, tunicamycin (TM). Electron microscopy data suggested that Mfn2 siRNA significantly disrupted ER-mitochondria tethering in ER stress-injured HL-1 cells. Mfn2 silencing attenuated mitochondrial oxidative stress and Ca2+ overload, increased mitochondrial membrane potential and mitochondrial oxygen consumption, and protected cells from TM-induced apoptosis. In summary, Mfn2 plays an important role in high glucose-induced ER stress in atrial cardiomyocytes, and Mfn2 silencing prevents mitochondrial Ca2+ overload-mediated mitochondrial dysfunction, thereby decreasing ER stress-mediated cardiomyocyte cell death.
Copyright © 2020 Ming Yuan et al.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
IP3R1/GRP75/VDAC1 complex mediates endoplasmic reticulum stress-mitochondrial oxidative stress in diabetic atrial remodeling.Redox Biol. 2022 Jun;52:102289. doi: 10.1016/j.redox.2022.102289. Epub 2022 Mar 21. Redox Biol. 2022. PMID: 35344886 Free PMC article.
-
Entresto protected the cardiomyocytes and preserved heart function in cardiorenal syndrome rat fed with high-protein diet through regulating the oxidative stress and Mfn2-mediated mitochondrial functional integrity.Biomed Pharmacother. 2021 Dec;144:112244. doi: 10.1016/j.biopha.2021.112244. Epub 2021 Oct 1. Biomed Pharmacother. 2021. PMID: 34601193
-
Loss and gain of function of Grp75 or mitofusin 2 distinctly alter cholesterol metabolism, but all promote triglyceride accumulation in hepatocytes.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Dec;1866(12):159030. doi: 10.1016/j.bbalip.2021.159030. Epub 2021 Aug 20. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 34419589
-
Mitochondrial dynamism and cardiac fate--a personal perspective.Circ J. 2013;77(6):1370-9. doi: 10.1253/circj.cj-13-0453. Epub 2013 Apr 25. Circ J. 2013. PMID: 23615052 Review.
-
Functional implications of mitofusin 2-mediated mitochondrial-SR tethering.J Mol Cell Cardiol. 2015 Jan;78:123-8. doi: 10.1016/j.yjmcc.2014.09.015. Epub 2014 Sep 22. J Mol Cell Cardiol. 2015. PMID: 25252175 Free PMC article. Review.
Cited by
-
Catechins as a Potential Dietary Supplementation in Prevention of Comorbidities Linked with Down Syndrome.Nutrients. 2022 May 12;14(10):2039. doi: 10.3390/nu14102039. Nutrients. 2022. PMID: 35631180 Free PMC article. Review.
-
Atrial Fibrillation in Endurance Training Athletes: Scoping Review.Rev Cardiovasc Med. 2023 May 26;24(6):155. doi: 10.31083/j.rcm2406155. eCollection 2023 Jun. Rev Cardiovasc Med. 2023. PMID: 39077536 Free PMC article.
-
Potential Roles of IP3 Receptors and Calcium in Programmed Cell Death and Implications in Cardiovascular Diseases.Biomolecules. 2024 Oct 20;14(10):1334. doi: 10.3390/biom14101334. Biomolecules. 2024. PMID: 39456267 Free PMC article. Review.
-
Ferroptosis, necroptosis and cuproptosis: Novel forms of regulated cell death in diabetic cardiomyopathy.Front Cardiovasc Med. 2023 Mar 10;10:1135723. doi: 10.3389/fcvm.2023.1135723. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36970345 Free PMC article. Review.
-
The Critical Roles of Proteostasis and Endoplasmic Reticulum Stress in Atrial Fibrillation.Front Physiol. 2022 Jan 4;12:793171. doi: 10.3389/fphys.2021.793171. eCollection 2021. Front Physiol. 2022. PMID: 35058801 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

