Methane-Rich Saline Alleviates CA/CPR Brain Injury by Inhibiting Oxidative Stress, Microglial Activation-Induced Inflammatory Responses, and ER Stress-Mediated Apoptosis
- PMID: 33149813
- PMCID: PMC7603629
- DOI: 10.1155/2020/8829328
Methane-Rich Saline Alleviates CA/CPR Brain Injury by Inhibiting Oxidative Stress, Microglial Activation-Induced Inflammatory Responses, and ER Stress-Mediated Apoptosis
Abstract
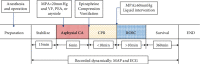
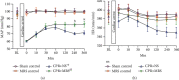
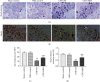
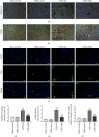
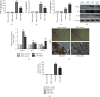
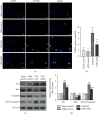
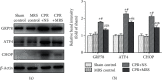
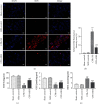
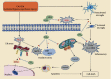
Brain injury induced by cardiac arrest/cardiopulmonary resuscitation (CA/CPR) is the leading cause of death among patients who have recovery of spontaneous circulation (ROSC). Inflammatory response, apoptosis, and oxidative stress are proven pathological mechanisms implicated in neuronal damage. Methane-rich saline (MRS) has been proven that exerts a beneficial protectiveness impact in several models of ischemia-reperfusion injury. The goal of this paper is to ascertain the role of MRS in CA/CPR-induced brain injury and its potential mechanisms. The tracheal intubation of Sprague-Dawley (SD) rats was clamped for 6 min to establish an asphyxiating cardiac arrest model. After that, chest compressions were applied; then, MRS or saline was administered immediately post-ROSC, the rats were sacrificed, and brain tissue was collected at the end of 6 hours. We observed that MRS treatment attenuated neuronal damage in the hippocampal CA1 region by inhibiting microglial activation, leading to a decrease in the overexpression of proinflammatory cytokines such as TNF-α, IL-6, and iNOS. The results also illustrated that MRS treatment diminished apoptosis in the hippocampal CA1 region , reduced the expression of apoptosis-associated proteins Bax and cleaved caspase9, and increased Bcl-2 expression, as well as inhibited the expression of endoplasmic reticulum (ER) stress pathway-related proteins GRP78, ATF4, and CHOP. Further findings showed that MRS treatment significantly attenuated hippocampal ROS and MDA levels and increased GSH and SOD antioxidant factor levels, which indicated that MRS treatment could inhibit oxidative stress. Our results suggest that MRS exerts a protective effect against CA/CPR brain injury, by inhibiting oxidative stress, microglial activation-induced inflammatory responses, and ER stress-mediated apoptosis.
Copyright © 2020 Ruixia Cui et al.
Conflict of interest statement
We declare that there is no conflict of interest regarding the publication of this article.
Figures
Similar articles
-
Inhibition of microglial activation contributes to propofol-induced protection against post-cardiac arrest brain injury in rats.J Neurochem. 2015 Sep;134(5):892-903. doi: 10.1111/jnc.13179. Epub 2015 Jun 11. J Neurochem. 2015. PMID: 26016627
-
Metformin ameliorates brain damage caused by cardiopulmonary resuscitation via targeting endoplasmic reticulum stress-related proteins GRP78 and XBP1.Eur J Pharmacol. 2021 Jan 15;891:173716. doi: 10.1016/j.ejphar.2020.173716. Epub 2020 Nov 13. Eur J Pharmacol. 2021. PMID: 33197442
-
Methane-Rich Saline Ameliorates Sepsis-Induced Acute Kidney Injury through Anti-Inflammation, Antioxidative, and Antiapoptosis Effects by Regulating Endoplasmic Reticulum Stress.Oxid Med Cell Longev. 2018 Nov 15;2018:4756846. doi: 10.1155/2018/4756846. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30581532 Free PMC article.
-
Upregulation of hemeoxygenase enzymes HO-1 and HO-2 following ischemia-reperfusion injury in connection with experimental cardiac arrest and cardiopulmonary resuscitation: Neuroprotective effects of methylene blue.Prog Brain Res. 2021;265:317-375. doi: 10.1016/bs.pbr.2021.06.009. Epub 2021 Aug 12. Prog Brain Res. 2021. PMID: 34560924 Review.
-
Pharmacological Approach for Neuroprotection After Cardiac Arrest-A Narrative Review of Current Therapies and Future Neuroprotective Cocktail.Front Med (Lausanne). 2021 May 18;8:636651. doi: 10.3389/fmed.2021.636651. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34084772 Free PMC article. Review.
Cited by
-
Hydrogen therapy after resuscitation improves myocardial injury involving inhibition of autophagy in an asphyxial rat model of cardiac arrest.Exp Ther Med. 2022 Jun;23(6):376. doi: 10.3892/etm.2022.11302. Epub 2022 Apr 7. Exp Ther Med. 2022. PMID: 35495584 Free PMC article.
-
Protective role of methane in traumatic nervous system diseases.Med Gas Res. 2024 Sep 1;14(3):159-162. doi: 10.4103/mgr.mgr_23_23. Epub 2024 Mar 20. Med Gas Res. 2024. PMID: 40232698 Free PMC article. No abstract available.
-
The ACE2/Ang-(1-7)/MasR axis alleviates brain injury after cardiopulmonary resuscitation in rabbits by activating PI3K/Akt signaling.Transl Neurosci. 2024 Apr 11;15(1):20220334. doi: 10.1515/tnsci-2022-0334. eCollection 2024 Jan 1. Transl Neurosci. 2024. PMID: 38623573 Free PMC article.
-
Targeting iNOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation.Mol Neurobiol. 2022 May;59(5):3124-3139. doi: 10.1007/s12035-022-02788-5. Epub 2022 Mar 9. Mol Neurobiol. 2022. PMID: 35262869
-
PD98059 protects SH-SY5Y cells against oxidative stress in oxygen-glucose deprivation/reperfusion.Transl Neurosci. 2023 Sep 6;14(1):20220300. doi: 10.1515/tnsci-2022-0300. eCollection 2023 Jan 1. Transl Neurosci. 2023. PMID: 37719747 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

